(UroToday.com) The 2023 SNMMI annual meeting included a prostate cancer session, featuring a presentation by Dr. Thomas Huettmann discussing the impact of higher SUV mean in baseline 18F-PSMA 1007 PET and the association with survival in patients undergoing radioligand therapy with [177Lu]Lu-PSMA I&T.
There is increasing evidence that quantified 68Ga-PSMA PET signal can identify high-risk prostate cancer patients scheduled for radioligand therapy. As such, the aims of the current study were as follows:
- Assess the prognostic potential of [18F]-PSMA-1007 PET for identifying responders scheduled for [177Lu]Lu-PSMA I&T
- Assess the prognostic potential of [18F]-PSMA-1007 PET for overall survival relative to established clinical parameters
- Provide a risk factor model including imaging and clinical items available at the time of treatment planning
In this retrospective study, 105 mCRPC patients treated with [177Lu]Lu-PSMA I&T were analyzed. The median age at the first cycle of radioligand therapy was 71 years, and a median of 3 cycles of radioligand therapy were administered, with a median activity of 14.4 GBq (range: 4.8-50.9) [177Lu]Lu-PSMA I&T. Whole body tumor burden derived from baseline [18F]-PSMA 1007 PET was quantified, including SUVmean, SUVmax, PSMA tumor volume and total lesion PSMA (total lesion-PSMA = PSMA tumor volume*SUVmean). Baseline laboratory values (hemoglobin, C-reactive protein, lactate dehydrogenase, aspartate aminotransferase and alkaline phosphatase) were also collected. Analyses included univariable cox regression analysis, followed by multivariable and Kaplan Meier analyses.
Median overall survival in this cohort was 16 months. Univariable analysis provided the following significant parameters: SUV mean, liver metastases, C-reactive protein, lactate dehydrogenase und hemoglobin:
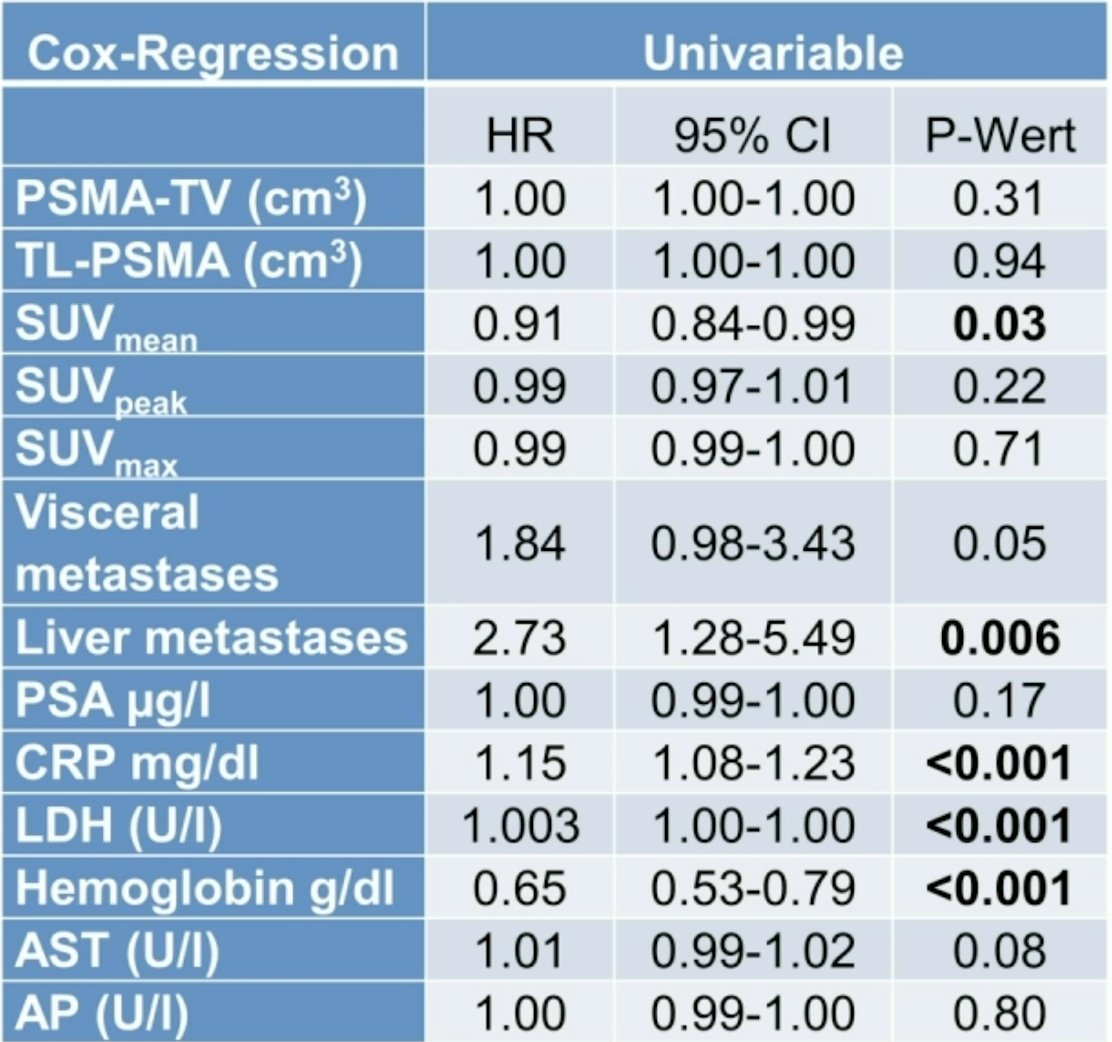
On multivariable cox regression analysis, significant factors as prognostic for overall survival included: baseline SUV mean (per unit, HR 0.91, 95% CI 0.83-0.99), liver metastases (HR 2.37, 95% CI 1.05-5.13), C-reactive protein (per mg/dl, HR 1.13, 95% CI 1.04-1.22), and hemoglobin (per g/dl, HR 0.76, 95% CI 0.61-0.93). Using a median SUVmean of 9.4, Kaplan Meier analyses revealed significant segregation between individuals with SUV mean below (9 months) or above the median (19 months, HR 1.76, p = 0.03):
![[177Lu]Lu-PSMA I&T SUV](/images/com-doc-importer/127-snmmi-2023/snmmi-2023-higher-suv-mean-in-baseline-18f-psma-1007-pet-is-associated-with-longer-survival-in-patients-undergoing-radioligand-therapy-with-177lu-lu-psma-i-t/image-1.jpg)
Presence of liver metastases was also associated with poorer survival (median 6 months) compared to those without liver metastases (median 17 months):
![[177Lu]Lu-PSMA I&T liver metastasis](/images/com-doc-importer/127-snmmi-2023/snmmi-2023-higher-suv-mean-in-baseline-18f-psma-1007-pet-is-associated-with-longer-survival-in-patients-undergoing-radioligand-therapy-with-177lu-lu-psma-i-t/image-2.jpg)
Additionally, an increased number of risk factors was associated with shorter survival in the risk factor model:

Dr. Huttmann concluded his presentation discussing the impact of higher SUV mean in baseline F18-PSMA 1007 PET and the association with survival in patients undergoing radioligand therapy with [177Lu]Lu-PSMA I&T with the following take home messages:
- Lower SUVmean is associated with shorter overall survival
- The SUVmean threshold in this study was virtually identical to the cut-off presented in the TheraP trial1
- Higher C-reactive protein, lower hemoglobin levels, and presence of liver metastases were independently associated with reduced overall survival
- In the risk factor model, increasing number of risk factors was associated with a worse outcome
Presented by: Thomas Huettmann, Klinik und Poliklinik fur Nuklearmedizin, Wuerzburg, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, Chicago, IL, Sat, June 24 – Tues, June 27, 2023.
Reference:


