(UroToday.com) The 2023 SNMMI annual meeting included a prostate cancer session, featuring a presentation by Dr. Isabel Rauscher discussing safety and efficacy of 177Lu-PSMA-I&T radioligand therapy in octogenarians with metastatic castration-resistant prostate cancer (mCRPC). Lutetium-177 PSMA radioligand therapy is a new treatment option for mCRPC and has received recent FDA approval based on the results of the VISION trial.1
Its low toxicity profile favors the use in elderly patients or in patients with critical comorbidities, however only 1.7% of patients in VISION were over the age of 85 years. Additionally, the proportion of octogenarians with advanced prostate cancer continues to increase. The objective of this study was to retrospectively analyze the efficacy and safety of 177Lu-PSMA-radioligand therapy in mCRPC patients ≥ 80 years of age, and assess response and toxicity rates in chemotherapy pre-treated compared to chemotherapy naïve patients.
There were 80 mCRPC patients ≥80 years of age (median age 82; range: 80-91 years) that underwent 177Lu-PSMA-I&T radioligand therapy and were retrospectively selected from Dr. Rauscher’s institutional database (October 2014 to February 2022). Eligibility criteria for 177Lu-PSMA-I&T radioligand therapy were in accordance with current guidelines. Patients had previously been treated with androgen receptor-directed therapy (abiraterone or enzalutamide), received taxane-based chemotherapy, or were chemotherapy-ineligible. All tumor lesions showed higher PSMA-ligand uptake than the liver at baseline PSMA-PET. Best PSA-response, clinical progression-free survival (defined as clinical progression, or progressive disease on subsequent PSMA-PET/CT or death) and overall survival were calculated, and toxicity data were acquired until 6 months after last treatment cycle.
Among these 80 patients, 49 (61.3%) were chemotherapy-naïve, 31 were chemotherapy pretreated, 16 (20%) had visceral metastases, and the median number of previous mCRPC treatment regimens was 2. A total of 324 cycles (median 4 cycles, range: 1–12) with a median cumulative activity of 23.8 GBq (IQR: 14.8-42.2) were administered every 4 to 6 weeks. A PSA decline of 50% was achieved in 37 (46.3%) patients, and any PSA decline was achieved in 67.5% of patients. Chemotherapy-naïve patients showed higher 50% PSA response rates compared to chemotherapy-pretreated patients (51.0% vs. 38.7%):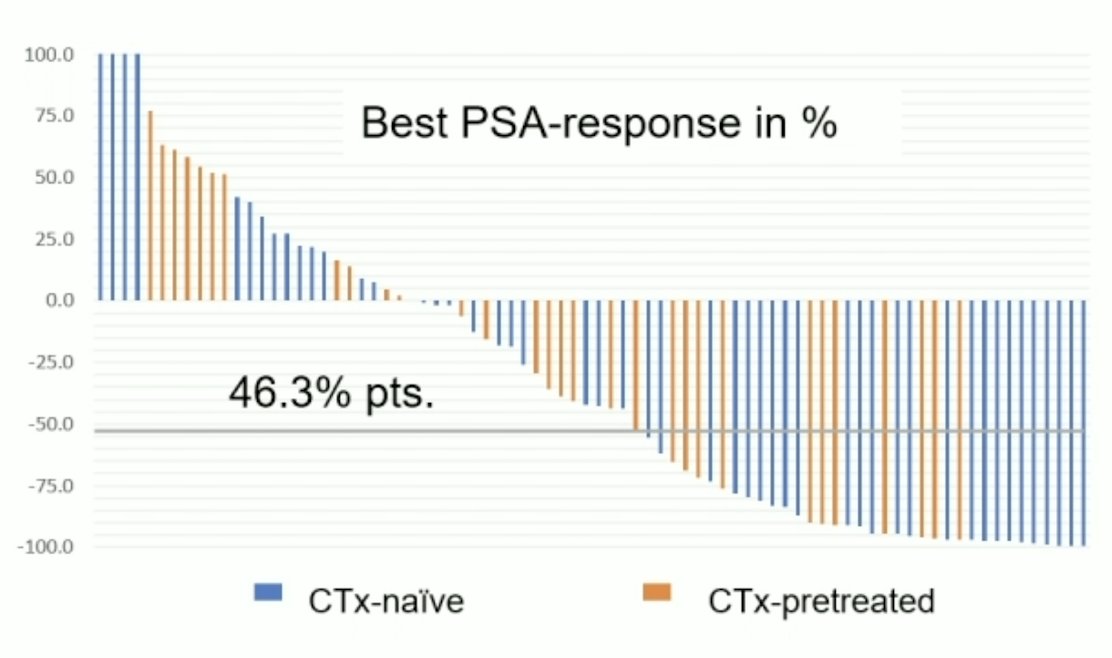
Overall, median clinical progression-free survival and overall survival were 8.7 and 16.1 month, respectively. Median clinical progression-free survival and overall survival of chemotherapy-naïve patients were significantly longer compared to chemotherapy-pretreated patients (10.5 vs. 6.5 month and 20.7 vs. 11.8 mo, respectively, p<0.05):
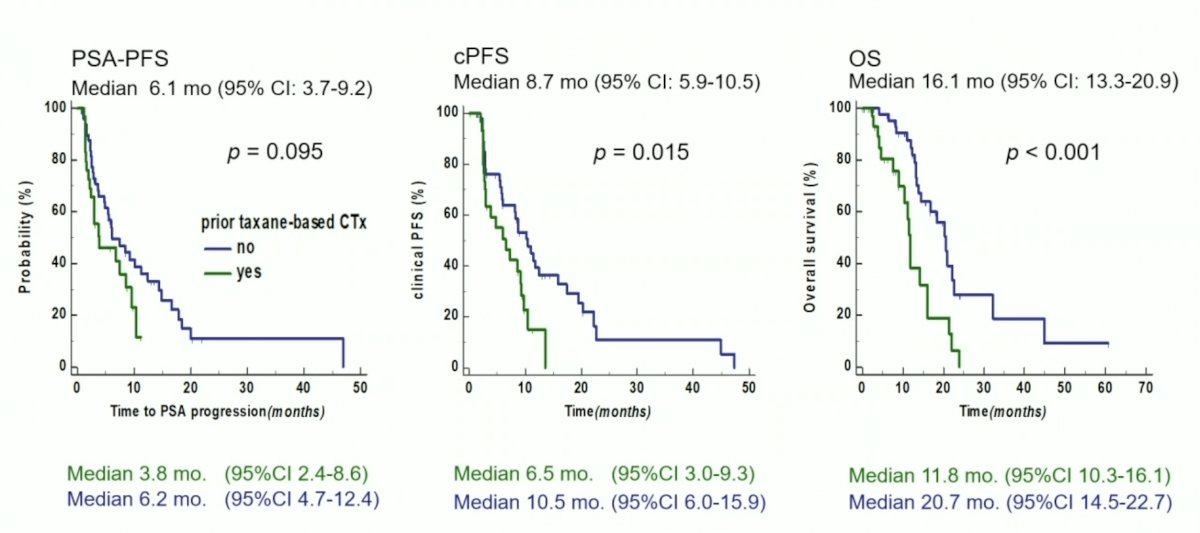
Lower hemoglobin level and higher LDH level at baseline were independent predictors of poorer clinical progression-free survival and overall survival. Treatment-emergent grade 3 toxicities were anemia in 4 (5%), thrombocytopenia in 3 (3.8%) and renal impairment in 4 (5%) patients. No non-hematologic grade 3 and no grade 4 toxicities were observed. Most frequent clinical side effects were grade 1-2 xerostomia, fatigue and loss of appetite:

ECOG performance status improved in 5/80 patients, was stable in 56/80 patients, and worsened in 18/80 patients. Limitations of this study include the retrospective design, single institutional data, and limited patient number.
Dr. Rauscher concluded her presentation discussing safety and efficacy of 177Lu-PSMA-I&T radioligand therapy in octogenarians with mCRPC with the following take home messages:
- 177Lu-PSMA-I&T radioligand therapy in elderly mCRPC patients ≥80 years is safe and effective, comparable to previously published data of non-age selected cohorts with a low rate of high-grade toxicities
- VISION trial1: median progression free survival 8.7 months, median overall survival 15.3 months
- Current study: median clinical progression free survival 8.7 months, median overall survival 16.1 months
- Chemotherapy-naïve patients constituting the majority of this cohort showed a better and longer response to therapy than taxane-pretreated patients
- Tendency towards better PSA response, longer clinical progression free survival and overall survival compared to chemotherapy pretreated patients
- Robust data is awaited from the PSMAFore trial
- 177Lu-PSMA radioligand therapy seems to be a meaningful treatment option for older patients
Presented by: Isabel Rauscher, Technical University of Munich, Munich, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, Chicago, IL, Sat, June 24 – Tues, June 27, 2023.
References:


