(UroToday.com) The 2023 SNMMI annual meeting included a prostate cancer session, featuring a presentation by Dr. Harshad Kulkarni discussing personalized PSMA-directed molecular radiotherapy with identification of favorable genomic and post-therapeutic radiomic parameters. With the publication of the VISION trial, the FDA and EMA subsequently approved the use of Lu-177 PSMA-617 in the third line setting of metastatic castration-resistant prostate cancer (mCRPC) treatment.1
However, the genomic profile of these patients is currently under studied. As such, the objective of this retrospective study was to assess the outcome of treatment using Lu-177 PSMA-617 in mCRPC patients and to identify genomic features and post-therapeutic SPECT/CT parameters as predictors of response.
Between August 3, 2022, and June 15, 2023, there were 88 patients with mCRPC included in this study. The median age was 62 years (range: 49 – 93) and median Gleason score was 8 (range: 7-10). These patients were treated with 1-6 cycles (262 in all) of treatment using Lu-177 PSMA-617 (5.9 – 7.4 GBq) according to NCCN guidelines and after prior confirmation of PSMA expression on PSMA-directed PET. With a median interval of 6 weeks between cycles (up to 9 weeks), 8 patients underwent 6 cycles, 7 underwent 5 cycles, and 14 had 4 cycles, whereas 20 patients received 2 cycles and 12 patients received 1 cycle of treatment. Hematological status, renal and hepatic function and serum PSA levels were documented before and after therapy. All patients underwent post-therapeutic SPECT/CT at one time-point 1-4 days after treatment to assess post-therapeutic biodistribution and assess molecular therapy response. The indices maximum standardized uptake value (SUVmax), SUVmean, and molecular tumor index (molecular tumor volume*SUVmean) were analyzed post-Lu-177 PSMA-617 and compared with the percentage change in PSA from baseline until time of best PSA response.
Early identification of progression based on PSA and SPECT/CT resulted in discontinuation of treatment after 2 cycles in 12 patients, and after 3 cycles in 3 patients. Due to a significant reduction in tumor burden and uptake on post-therapeutic SPECT/CT, as well as PSA response, 2 patients required only 2 cycles, whereas 13 patients required 3 treatment cycles. The treatment was well tolerated in all patients except 4, who had reversible nausea and vomiting. Grade 3 anemia and pancytopenia was observed in two patients, requiring discontinuation, however no significant (ie. grade 3-4 hematological/organ toxicity) was observed. This was true also in 3 patients with prior renal disease and 2 patients with a history of bone-marrow dysfunction, who were administered a reduced Lu-177 PSMA-617 radioactivity (5.9 GBq). The frequency of ATM mutations was maximum in the responders, present in 6/15 patients (40%) with an exceptional response after 2-3 cycles:

Interestingly, 2/4 of patients having vomiting had ATM mutations and 3/4 of patients were responders having a residual low-volume disease. Because percentage changes in PSA were not normally distributed, Spearman’s rank correlation analysis was not used to test for correlation. Additionally, the percentage reductions from the first SPECT/CT scan to the second SPECT/CT scan for SUVmax, SUVmean, and molecular tumor index were found to be statistically significant:

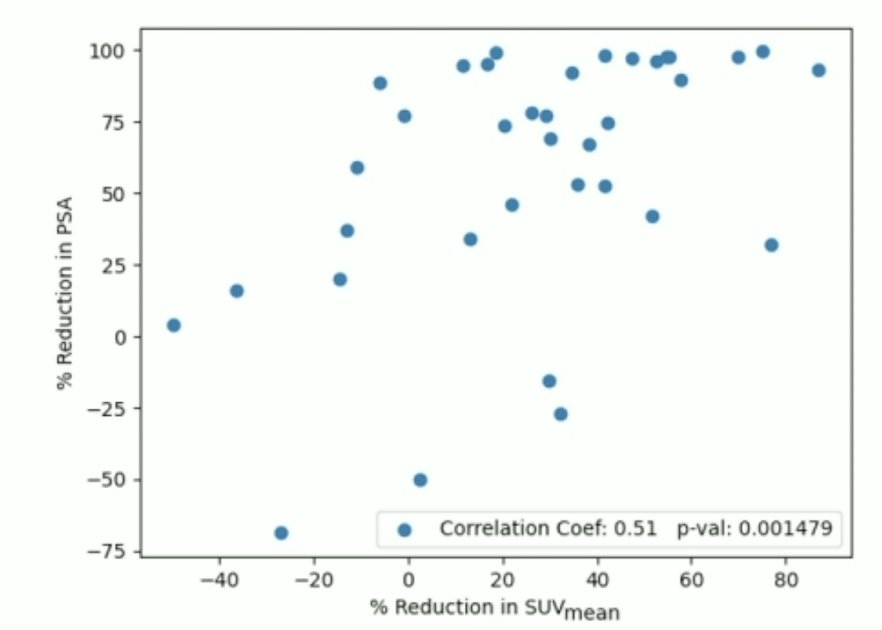
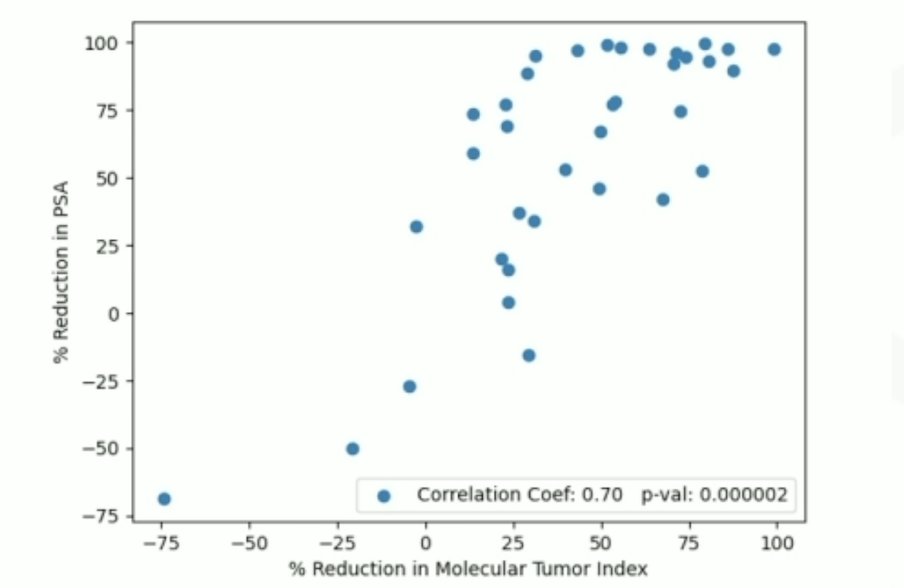
Dr. Kulkarni concluded his presentation discussing personalized PSMA-directed molecular radiotherapy with identification of favorable genomic and post-therapeutic radiomic parameters with the following take home messages:
- Analysis of genomic features could assist in predicting response to Lu-177 PSMA-617
- ATM mutations appear to be associated with a favorable response
- Reduction in molecular tumor index on SPECT/CT correlated best with PSA response and may be used as a prognostic radiomics biomarker
- Future prospective clinical trials will examine the integration of genomic and radiomics features, in concert with patient and disease specific factors, for the personalization of therapy
Presented by: Harshad R. Kulkarni, MD, BAMF Health, Grand Rapids, MI
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, Chicago, IL, Sat, June 24 – Tues, June 27, 2023.
Reference:


