(UroToday.com) The 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting held in Chicago, IL between June 24th and 27th, 2023 was host to a prostate cancer session. Dr. Angela Castellanos Rieger presented the results of an analysis evaluating the prognostic value of prostate-specific membrane antigen (PSMA) positron emission tomography (PET) among castrate-resistant prostate cancer (CRPC) patients in the pre-chemotherapy, post-androgen receptor signaling inhibitor (ARSI) treatment setting.
CRPC represents a heterogenous patient population with a limited life expectancy of approximately three years, when evaluated/treated within the context of clinical trials.1 There are currently no clear-cut recommendations for the most effective 1st line treatment regimen in this setting (docetaxel, ARSI, Radium-223). To date, there is limited data available on the role of PSMA-PET/CT for guiding treatment decisions in this setting. As such, the aim of this study was to evaluate the prognostic value of PSMA-PET for predicting treatment outcomes among CRPC patients, namely biochemical progression, radiographic progression, and overall survival.
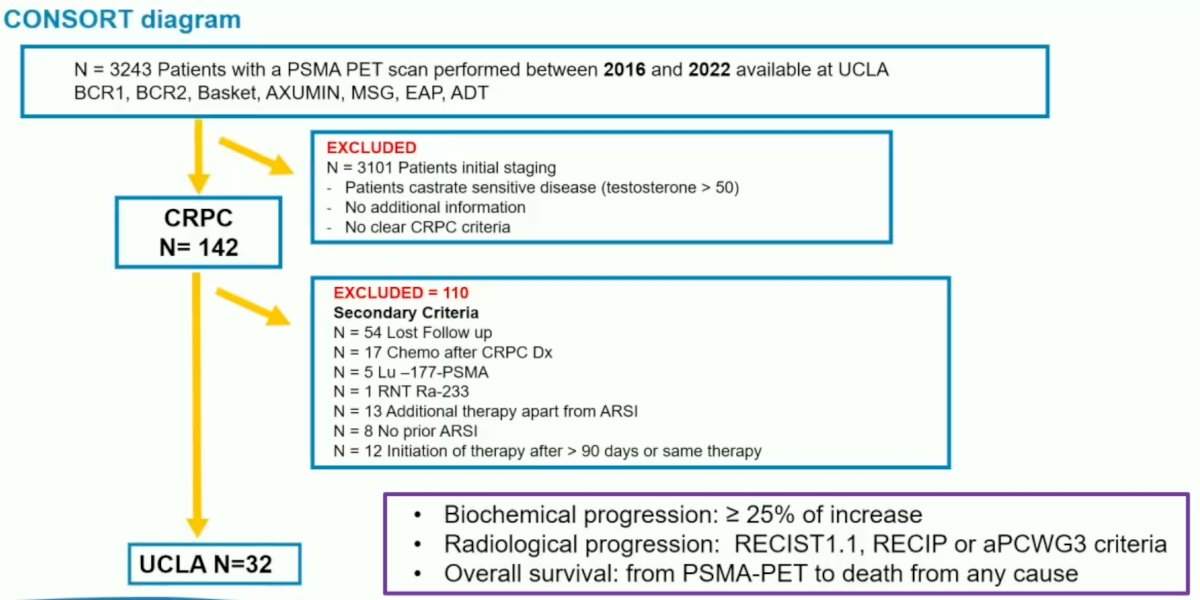
This study included patients with CRPC who had biochemical and imaging progression following prior treatment with 1-2 ARSIs. Patients did not receive any new therapy within 90 days of PSMA-PET/CT being performed and had at least 12 months of clinical follow-up available. Exclusion criteria were as follows:
- Testosterone >50 ng/dl
- Additional therapy not including androgen deprivation therapy (ADT), ARSI, or stereotactic body radiation therapy (SBRT)
- No prior chemotherapy for in the castrate-resistant setting (permitted during castrate-sensitive state)
- No prior radionuclide therapies (RNT)
Biochemical progression was defined as a ≥25% increase in the PSA level. Radiological progression was defined using RECIST 1.1, RECIP or aPCWG3 criteria. Overall survival was defined from the date of PSMA PET until death from any cause.
The authors identified 32 patients with CRPC who had a PSMA PET scan performed between 2016 and 2022 and who met all the study eligibility criteria.
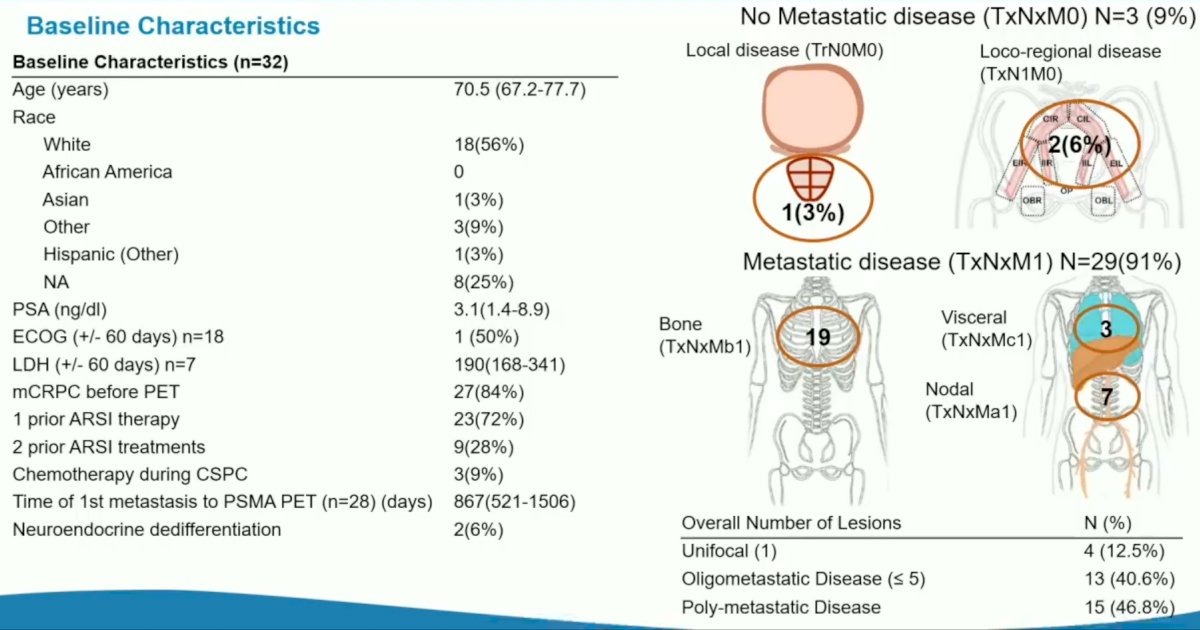
The median patient age was 70.5 years. 84% of the patients had mCRPC prior to PSMA PET and 72% had received treatment with one prior ARSI. 9% of patients received chemotherapy during the castrate-sensitive state. On PSMA PET, 3% had localized disease only, 6% had loco-regional disease, and 29/32 (91%) had evidence of metastatic disease (TxNxM1). Oligometastatic (5 or less) and polymetastatic disease was present in 41% and 47% of patients, respectively.
Radiographic progression occurred in 22/32 patients a median time of 343 days and was detected most commonly using a PSMA PET scan.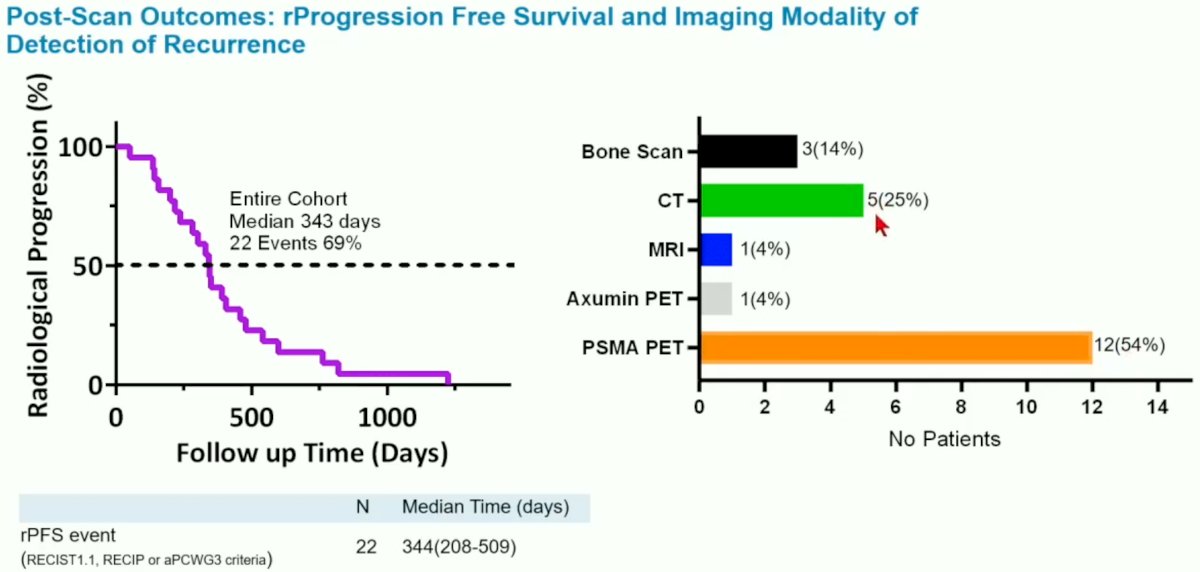
Biochemical progression occurred in 27/32 patients at a median follow-up of 280 days, with 7/32 patients dying at a median follow-up of 612 days.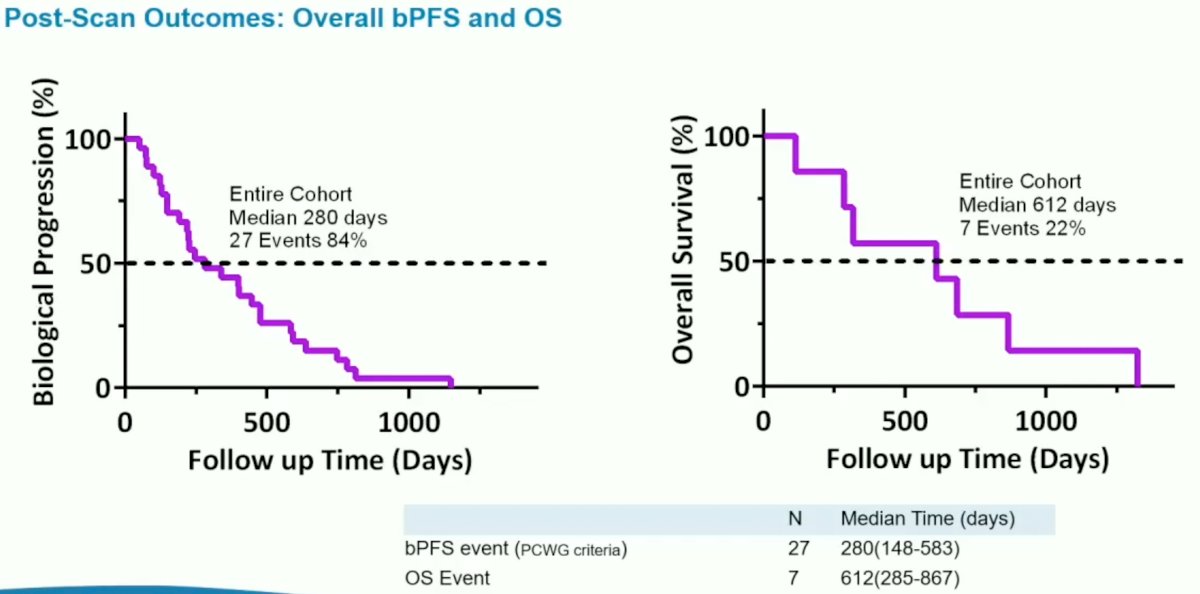
The median follow-up duration for the cohort was 664 days. The median time from PSMA PET to initiation of next line therapy was 33.5 days, with the most commonly used agent in this next line treatment setting being ARSI (19%), SBRT (19%), and RNT (16%).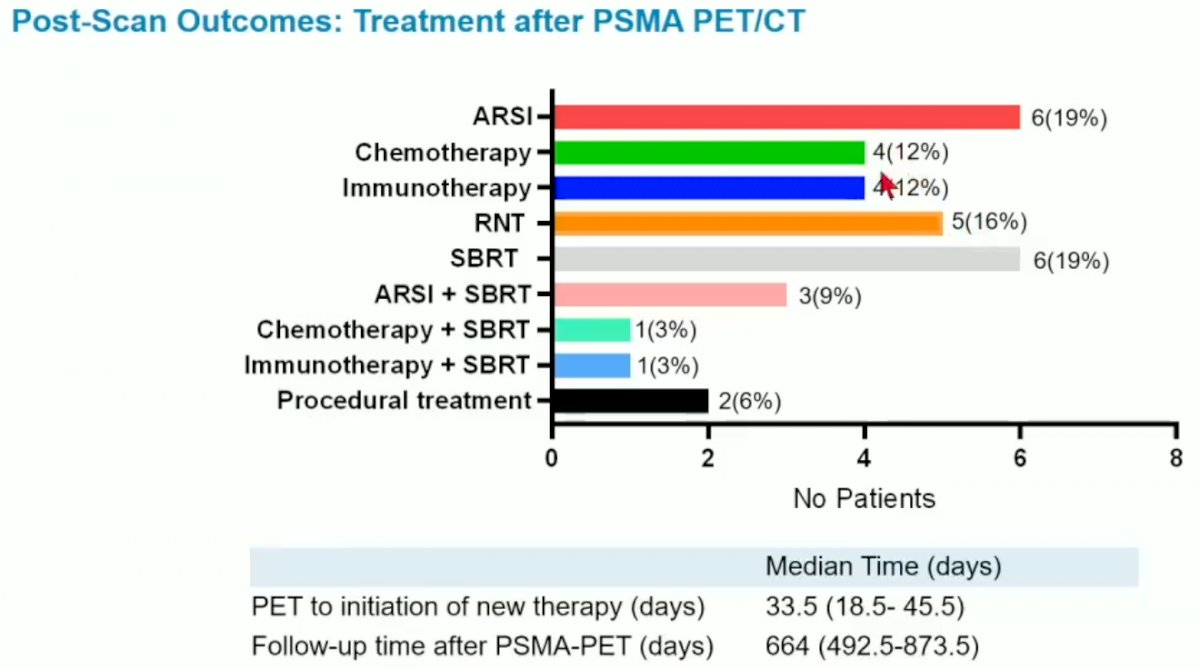
When outcomes were stratified by metastatic stage, the authors noted that radiologic progression (p=0.02), but not biochemical progression (p=0.06) or overall survival (p=0.4), was significantly superior among patients with lymph node metastases (M1a), compared to those with bone (M1b) or visceral (M1c) metastases.
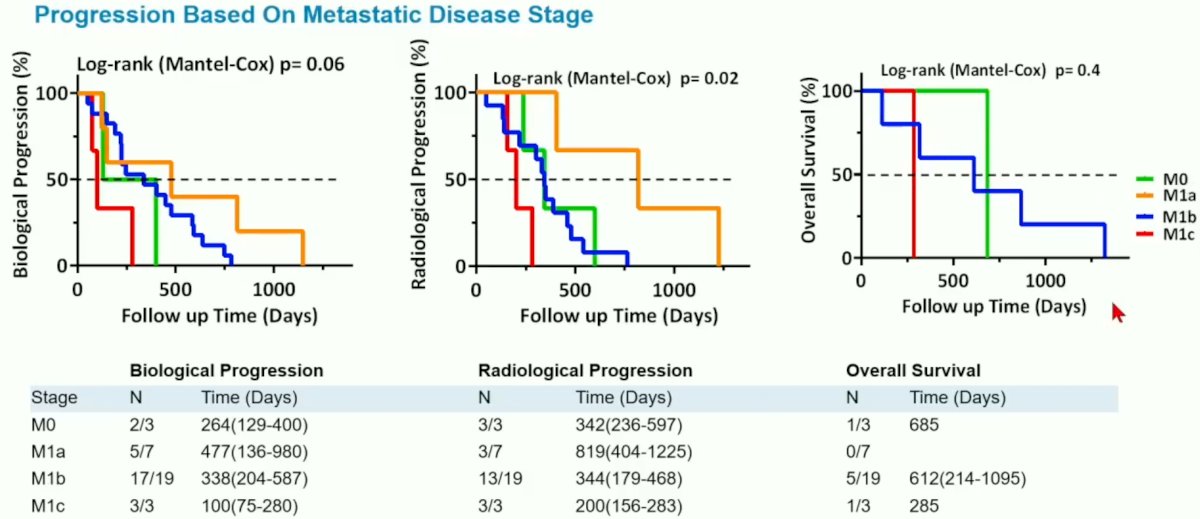
Based on these results, Dr. Rieger concluded that:
- PSMA-PET/CT can provide prognostic value for radiographic progression of CRPC patients following systemic therapy
- PSMA-PET/CT was not predictive of biochemical progression in this study, likely owing to the small sample size
- Limitations to this study include the small sample size and retrospective study design
Presented by: Angela Castellanos Rieger, MD, MSc, Resident Physician, Department of Radiology and Nuclear Medicine, University of California, Los Angeles, CA
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, Chicago, IL, Sat, June 24 – Tues, June 27, 2023.
Reference:- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368(2):138-48.


