(UroToday.com) The Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024 was host to a prostate cancer imaging session. Dr. John Nikitas presented an update from the PSMA-dRT trial, a randomized phase III trial of PSMA-PET/CT prior to definitive radiation therapy for unfavorable intermediate-risk or high-risk prostate cancer.
Prostate-specific membrane antigen (PSMA) is highly-expressed on prostate cancer cell surfaces, making it an attractive target for prostate cancer-directed radiotracers. PSMA-PET/CT offers superior diagnostic accuracy for nodal and distant metastases compared to conventional imaging and is now approved for the initial staging of select intermediate- and high-risk prostate cancer patients.1
The evidence for PSMA-PET/CT prior to definitive radiotherapy remains limited to non-randomized studies which have demonstrated that distant metastases are present in 6–9% of patients, and findings lead to radiation dose-escalation or pelvic lymph node irradiation in 13–20% of patients.2-4
PSMA-dRT is a multicenter, randomized phase III trial (NCT04457245) that was powered to randomize 312 men with unfavorable intermediate- or high-risk prostate cancer in a 1.08 to 1 ratio to receive a PSMA-PET/CT prior to definitive radiotherapy versus no PSMA-PET/CT. The 1.08 to 1 ratio was chosen given the assumption that 8% of patients in the PSMA-PET/CT arm would have evidence of extra-pelvic metastases and would be excluded.
The study inclusion criteria are as follows:
- Biopsy-proven prostate cancer
- Unfavorable intermediate- or high-risk disease:
- PSA ≥ 10 ng/mL
- or cT-stage≥2b
- or ISUP grade 3 (Gleason 4 + 3 = 7) or higher
- or ISUP grade 2 (Gleason 3 + 4 = 7) AND ≥ 50% positive biopsy cores
- or Decipher Score ≥ 0.45
- Intent for definitive radiotherapy
- Intent to incorporate PSMA-PET/CT findings into the radiotherapy plan if patient undergoes PSMA-PET/CT (intervention arm)
Exclusion criteria are as follows:
- Extra-pelvic metastasis (M1 disease) on any imaging or biopsy done prior to randomization
- Prior PSMA-PET/CT
- Prior pelvic radiotherapy
- Contraindications to radiotherapy (including active inflammatory bowel disease)
- Concurrent or prior surgery or systemic therapy for prostate cancer at the time of randomization.
In the intervention arm, patients were staged using a PSMA-PET/CT scan. In the control arm, patients were staged as per physician choice (CT, MRI, bone scintigraphy, or PET/CT with a non-PSMA radiotracer). The reports and Digital Imaging and Communications in Medicine (DICOM) images of the PSMA PET/CT were transferred to the treating radiation oncologist prior to radiotherapy planning.
With regards to treatment and follow-up, radiotherapy was delivered at the facility of the treating radiation oncologist, who decided on the radiation modality, dose and fractionation, inclusion of elective pelvic lymph nodes, and use of androgen deprivation therapy. Patients had follow-up visits every 3-4 months for the first year and every 6 months thereafter. Patients underwent serum PSA testing around the time of each follow-up visit. Imaging follow-up was ordered per the treating physician discretion if disease progression was suspected based on a rising PSA level.
The primary study endpoint was progression-free survival (PFS) at five years. Progression was defined by any of the following:
- PSA >2 ng/mL above post-radiotherapy nadir
- Recurrence on imaging or biopsy
- Initiation of salvage therapy
- All-cause death
PFS was estiamted using Kaplan-Meier survival analysis, with time-to-event analysis calculated from the time of randomization. Between-arm comparisons were performed using the log-rank test.
The U.S. Food and Drug Administration (FDA) approved PSMA-PET/CT radiotracers in 2021.
As a result, patients gained access to PSMA-PET/CTs as a standard, medically-reimbursed procedure. The recruitment rate significantly decreased, and most patients were receiving PSMA PET/CTs off-trial at the investigators’ institution. As a result, the trial closed prematurely in February 2022.
Between November 2020 and December 2021, 54 patients were randomized (PSMA-PET/CT arm: 25; control arm: 29). Two patients withdrew after randomization and were excluded (one from each arm). One patient had evidence of M1 disease on PSMA-PET/CT and was excluded from the primary analysis.

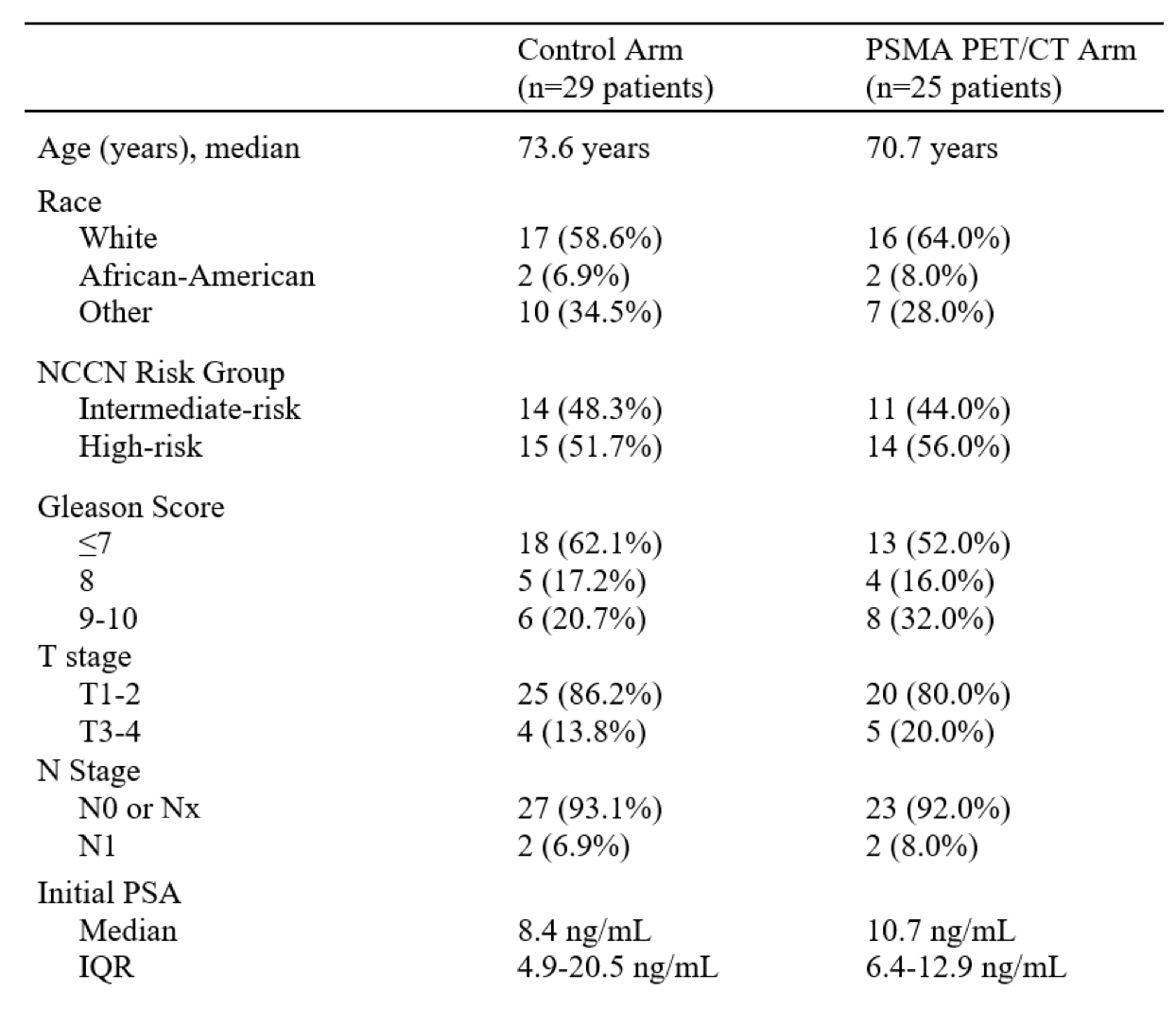
The baseline patient characteristics are summarized below. The median patient age was 70–74 years of age. 52–56% of patients had evidence of high-risk disease. 7–8% had evidence of N+ disease. The median PSA at baseline ranged between 8.4 and 10.7 ng/ml.
The PSMA-PET/CT results were as follows:
- 14 (58%) patients had localized disease (miT2b-cN0M0).
- 6 (25%) patients had locally-advanced disease (miT3a-bN0M0).
- 3 (13%) patients had regional metastases (miN1M0).
- 1 (4%) patient had regional and distant metastases (miN1M1b).
PSMA PET/CT upstaged four patients (16.7%), relative to baseline results:
- One with locally-advanced disease, two with regional metastases, and one with distant metastases.
- All three patients with miN1M0 disease received pelvic lymph node irradiation.
- The patient with miN1M1b disease received upfront ADT with abiraterone acetate plus prednisone followed by consolidative radiotherapy to the prostate and pelvic lymph nodes.
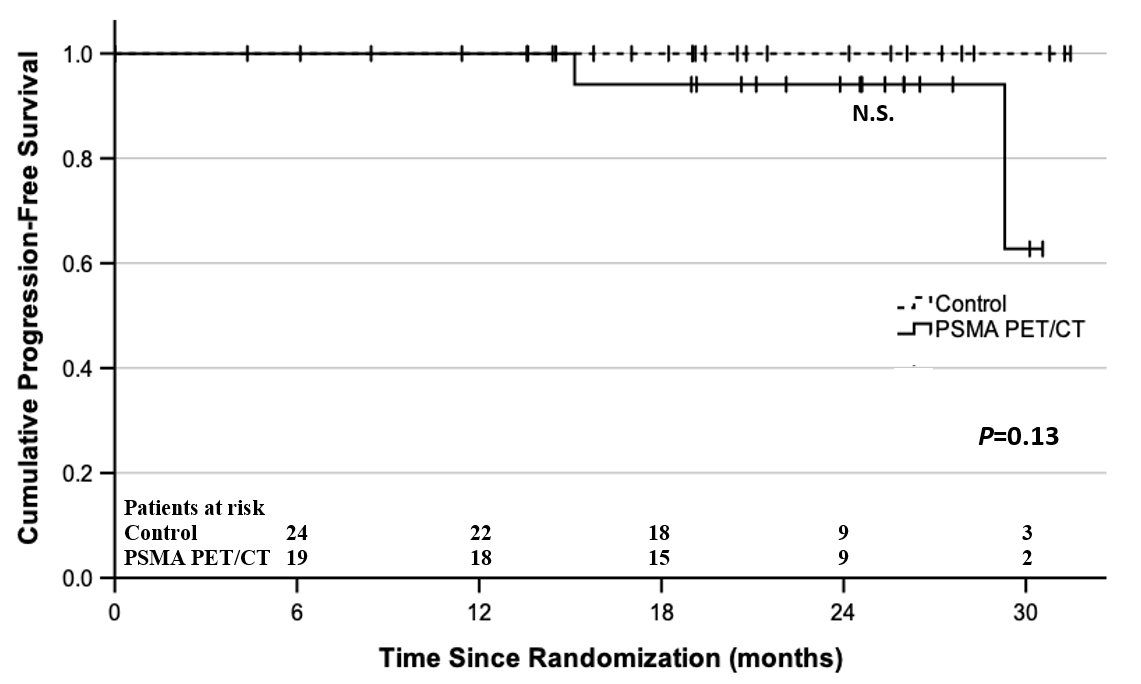
The median follow-up was 21 months (IQR: 17.6–26.3 months). There were no biochemical recurrence, disease recurrence, or prostate cancer mortality in either arm. There were two non-prostate cancer deaths in the PSMA-PET/CT arm. The two-year PFS was 93.8% for the PSMA-PET/CT arm and 100% for the control arm.
Dr. Nikitas concluded as follows:
- PSMA PET/CT upstaged one in six patients relative to baseline staging.
- This information guided target volume delineation, radiation dose, and hormone therapy intensification.
- This suggests that the adoption of PSMA PET/CT for primary staging in this patient population will impact treatment management choices.
- Unfortunately, this study was underpowered to detect a difference in progression-free survival due to early study termination.
- The window to conduct a randomized PSMA PET/CT trial and show long-term oncological benefits appears to be closing.
Presented by: John Nikitas, MD, Resident Physician, Department of Radiation Oncology, University of California, Los Angeles, CA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024
References:
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208-1216.
- Calais J, Zhu S, Hirmas N, et al. Phase 3 multicenter randomized trial of PSMA PET/CT prior to definitive radiation therapy for unfavorable intermediate-risk or high-risk prostate cancer [PSMA dRT]: study protocol. BMC Cancer. 2021;21: 512.
- Roach PJ, Francis R, Emmett L, et al. The Impact of 68Ga-PSMA PET/CT on Management Intent in Prostate Cancer: Results of an Australian Prospective Multicenter Study. J Nucl Med. 2018;59(1): 82-8.
- Sterzing F, Kratochwil C, Fiedler H, et al. (68)Ga-PSMA-11 PET/CT: a new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2016;43(1): 34–41.


