(UroToday.com) The Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024 was host to a prostate cancer imaging session. Dr. Rajender Kumar presented the results of a study evaluating percutaneous trans-gluteal Ga-68 PSMA PET/CT guided prostate biopsy in men with equivocal multiparametric MRI findings (i.e., PIRADS≤3 lesions).
The ESMO/EAU guidelines currently recommend performing a multiparametric MRI (mpMRI) in men with suspected prostate cancer prior to a prostate biopsy. However, a significant proportion of men with equivocal mpMRI findings (i.e, PIRADS ≤3) may harbor clinically significant disease, which may be missed if biopsy is limited to patients with PIRADS 4–5 disease.
There has been increased interest in evaluating 68Ga PSMA PET/CT in the primary diagnostic setting, particularly among men with a prior negative biopsy. In this prospective study, Dr. Kumar and colleagues evaluated the value of 68Ga PSMA-PET/CT-guided prostate biopsy in participants with PIRADS ≤3 lesions.
In this prospective study, participants meeting all of the following criteria were recruited and underwent whole-body 68Ga PSMA-PET/CT imaging:
- Lower urinary tract symptoms/abnormal digital rectal examination
- Elevated serum PSA >4 ng/ml
- Equivocal findings on mpMRI (i.e., PIRADS ≤3 lesions)
Exclusion criteria were as follows:
- Platelet counts <80,000
- Acute prostatitis or positive urine culture
- Metallic stent in situ
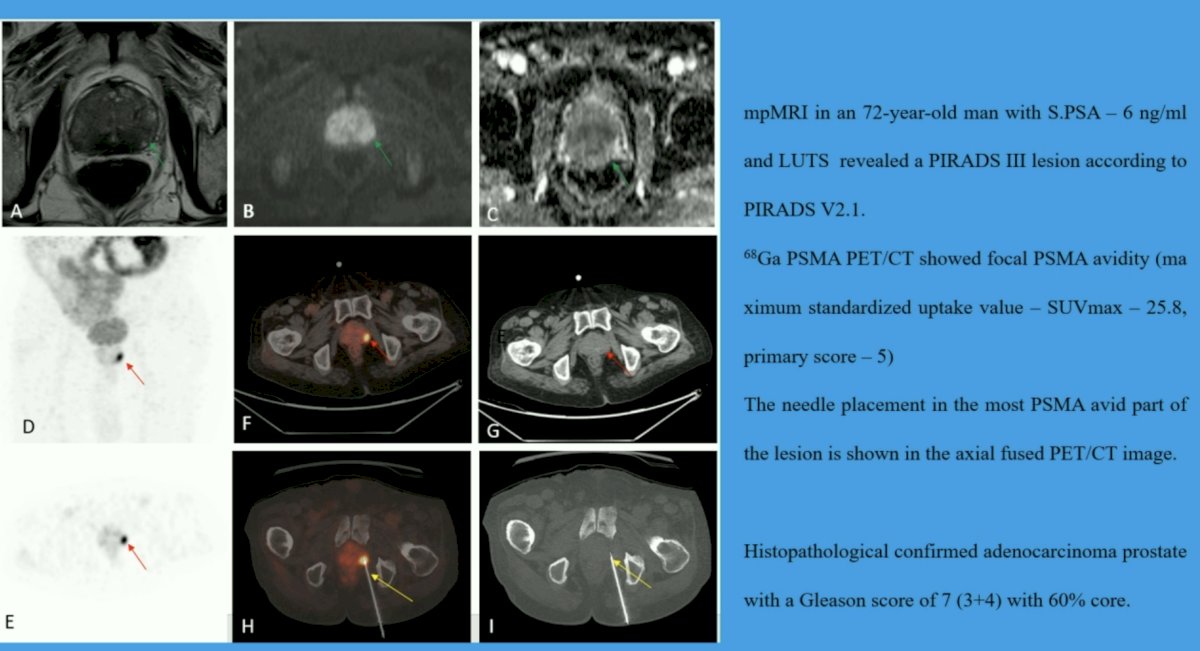
Two qualified nuclear medicine physicians reviewed the PET/CT imaging. A focal PSMA avid lesion in the prostate was considered PET-positive. Non-avid or heterogenous PSMA expression was considered PET negative and patients did not undergo a biopsy. The PET-positive patients underwent PET-guided prostatic biopsies through the trans-gluteal approach using an automated robotic arm to guide needle placement for prostatic lesion targeting. Society of Interventional Radiology consensus guidelines were followed for the procedures. Data regarding the location of tracer uptake in the prostate, SUVmax, visual analysis score (VAS) for pain, procedure-related complications, and histopathology with ISUP grade were collected. All patients had urine cultures performed 48 hours following the biopsy procedure.
A total of 50 participants were prospectively enrolled. The mean PSA level at enrollment was 12 ng/ml (range 4.3 to 19.7 ng/ml). Prostatic PSMA avid lesions were detected in 21/50 (42%) study participants. The mean SUVmax of the PET-positive lesions was 19.1 +/- 13.9. All PET-positive patients underwent PET/CT-guided prostatic biopsies, which were technically feasible in all 21 patients and all specimens retrieved were adequate for pathological analysis. Of note, one patient had a non-representative sample and required a repeat biopsy. The overall diagnostic yield was 100%. Intermediate-risk prostate cancer was diagnosed in 10 (20%) patients. Eleven (22%) patients were diagnosed with low-risk prostate cancer.
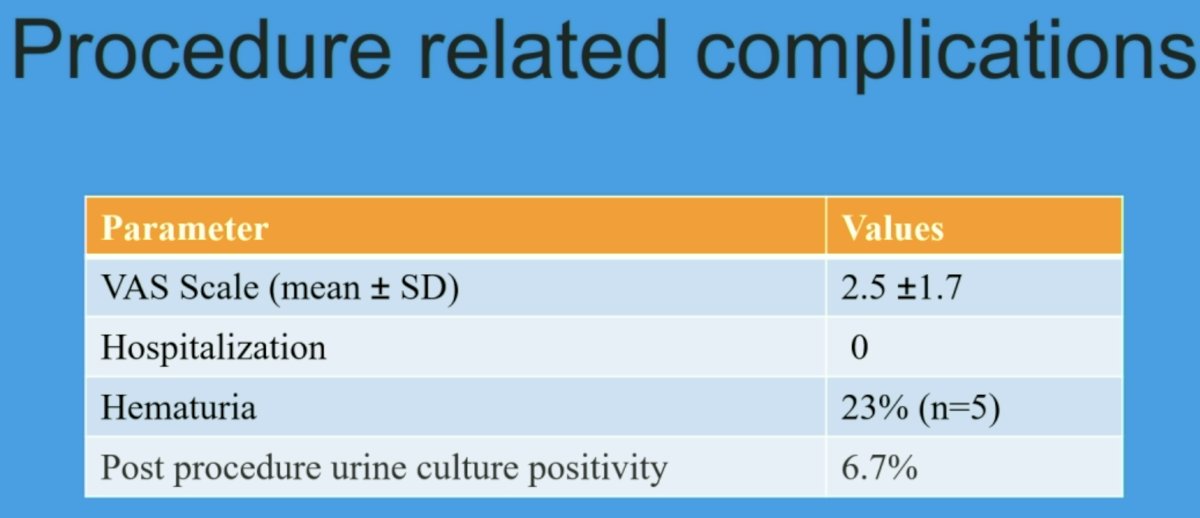
The mean VAS was 2.5 ±1.7 (> 5 in three). Minor complications (gluteal pains and hematuria) were noted in five patients. None of the patients required hospital admission. None of the patients developed fever or post-procedural infection. None of the PET-negative participants with a PIRADS ≤3 lesion has yet to be diagnosed with prostate cancer on follow-up.
Dr. Kumar concluded as follows:
- The present study demonstrates that many clinically significant prostate cancer lesions are missed when prostate biopsy is limited to patients with PIRAD >3 lesions on mpMRI.
- PSMA PET/CT-guided biopsy is a promising approach to diagnose prostate cancer in participants with equivocal mpMRI findings and a clinical suspicion for prostate cancer.
- This study suggests that a biopsy can be avoided for patients with PIRADS ≤3 lesions and no PSMA avid lesion, with none diagnosed with prostate cancer during the study follow-up period.
Presented by: Rajender Kumar, Department of Nuclear Medicine, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.


