(UroToday.com) The Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024 was host to a prostate-specific membrane antigen (PSMA) radioligand therapy (RLT) tumor board session. Dr. Michael Hofman discussed patient eligibility for RLT, highlighting the patient populations most likely to benefit from these targeted therapies.
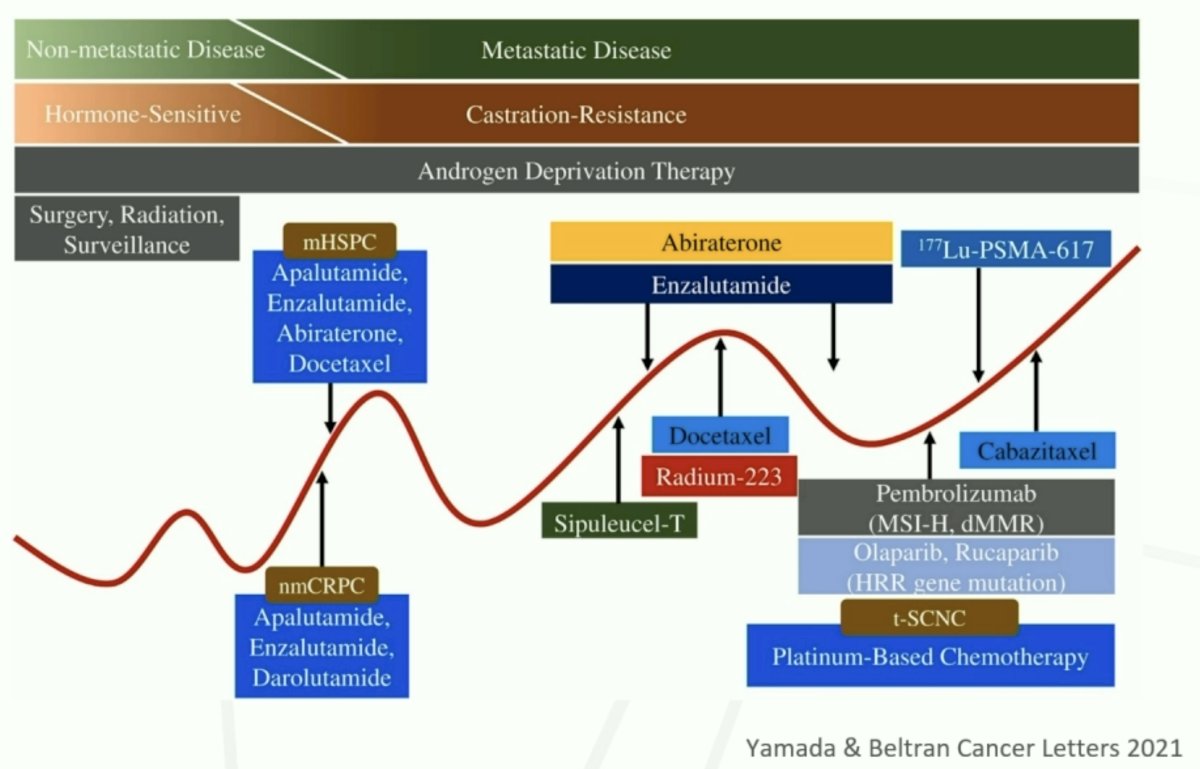
177Lu-PSMA-617 (PLUVICTO®) is currently approved for the treatment of adult metastatic castrate-resistant prostate cancer (mCRPC) patients who have disease progression following treatment with an androgen receptor pathway inhibitor (ARPI) and taxane-based chemotherapy.1 Dr. Hofman noted that 177Lu-PSMA-617 may be considered in patients who are medically unsuitable for 1st line chemotherapy (although not approved in this setting) and prior to 2nd line chemotherapy (e.g., cabazitaxel).
One of the unique features of radioligand therapy with 177Lu-PSMA-617 is that PSMA-PET/CT allows for disease visualization, assessment of tumor volume, and prediction of likelihood of response.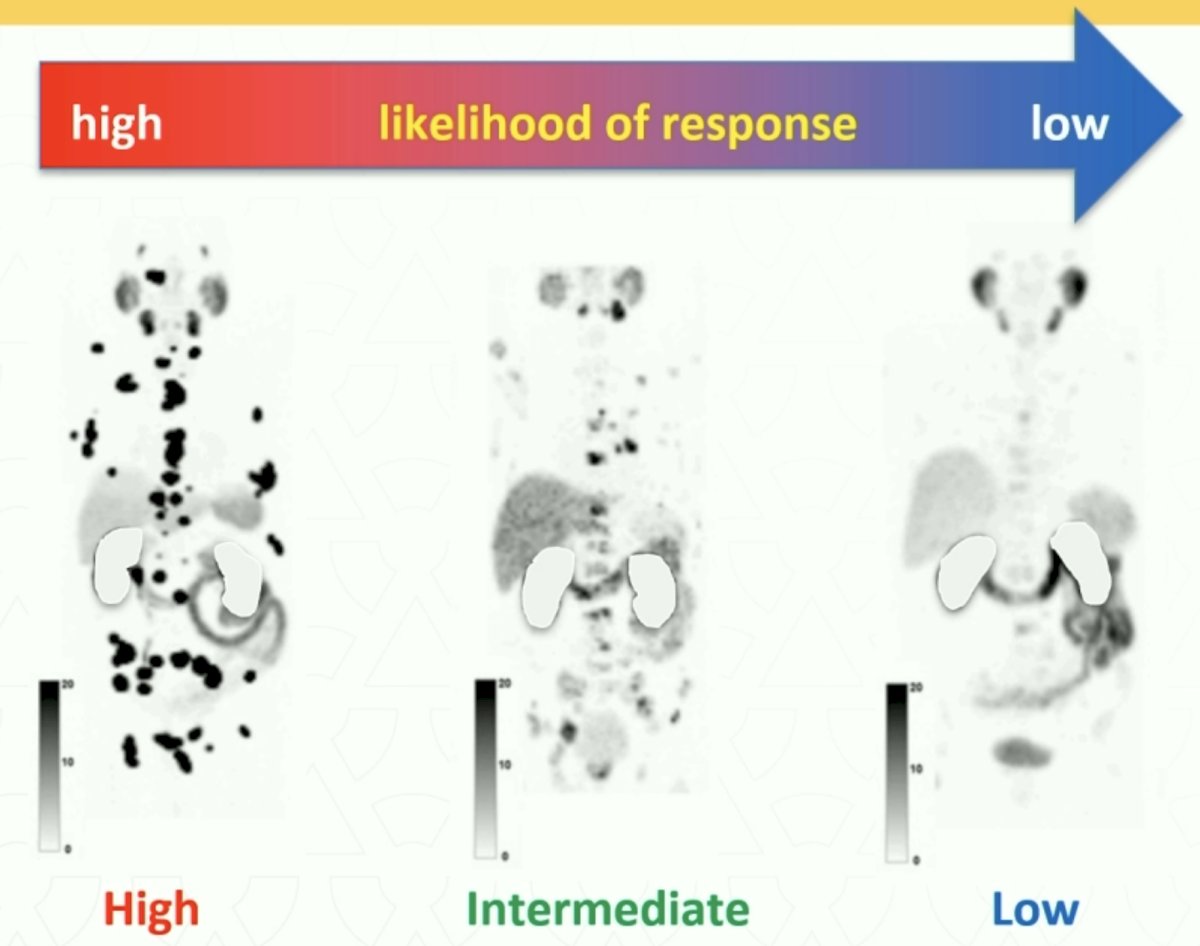
There are two major trials of 177Lu-PSMA-617 in the mCRPC disease space: VISION and TheraP.2,3

VISION is an international, open-label, phase 3 trial that evaluated 177Lu-PSMA-617 in mCRPC patients previously treated with an ARPI and 1-2 taxane regimens and who had PSMA-positive 68Ga-PSMA-PET/CT scans. Between June 2018 and October 2019, 831 patients were randomly assigned in a 2:1 ratio to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks for four to six cycles) plus protocol-permitted standard care or standard care alone. At a median follow-up of 20.9 months, 177Lu-PSMA-617 plus standard care significantly prolonged, as compared with standard of care, both radiographic progression-free survival (rPFS; median: 8.7 versus 3.4 months; HR: 0.40, p<0.001) and overall survival (median: 15.3 versus 11.3 months; HR: 0.62; 95% CI: 0.52 to 0.74, p<0.001). While a higher rate of high-grade (grade 3-5) treatment-emergent adverse events was observed with 177Lu-PSMA-617 (28.4% versus 3.9%) at the time of initial reporting, overall therapy was well tolerated. It bears note that treatment exposure was more than three times longer in the 177Lu-PSMA-617 group than in the control group.
TheraP was an open-label, phase II trial of 200 mCRPC men who were randomized to either 177Lu-PSMA-617 or cabazitaxel. To screen into the study, all men had both 68Ga-PSMA-11 and 18F-FDG PET/CT and were required to have high PSMA-expression (≥1 site with SUVmax ≥20) and no sites of FDG-positive/PSMA-negative disease. All patients had progressive disease with rising PSA ≥20 ng/mL after docetaxel and 91% had received prior enzalutamide or abiraterone. 200 patients were randomized 1:1 to 177Lu-PSMA-617 6-8 GBq every 6 weeks for up to 6 cycles of therapy or cabazitaxel 20 mg/m2 every three weeks for up to 10 cycles.
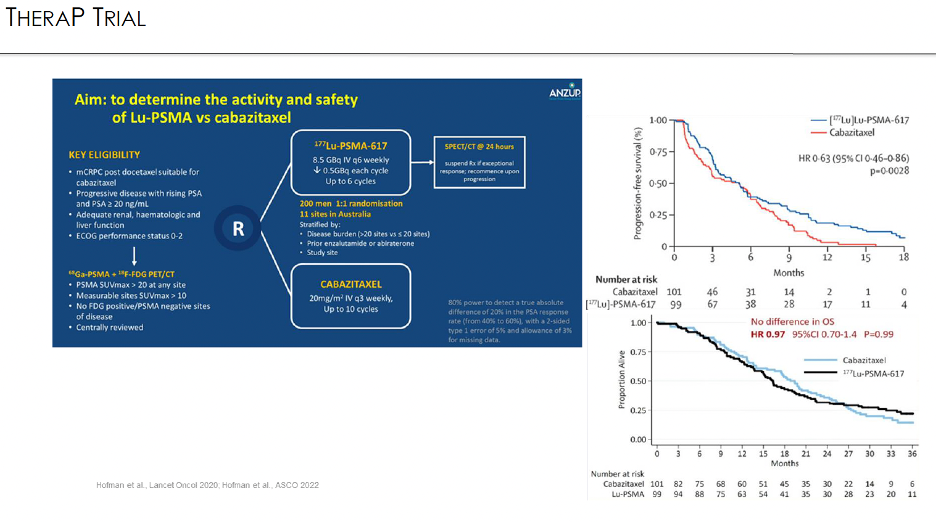
There are important differences in the selection criteria between the two trials. All patients in TheraP underwent both a 68Ga-PSMA-11 PET/CT + 18F-FDG-PET/CT, which was not mandated by the VISION trial. All TheraP trial participants were required to have a PSMA SUVmax ≥20, SUVmax >10 at sites of measurable soft tissue disease, and no FDG/PSMA-discordant lesions. In contrast, VISION-eligible patients were required to have disease PSMA uptake that was visually higher than that of the liver (~SUVmax >5–7) and no PSMA-negative metastases on CT.

These selection criteria were reflected in a larger proportion of screened patients being deemed trial-ineligible in the TheraP trial (28% versus 12% in VISION), with higher PSA50 response rates observed likely owing to these more stringent eligibility criteria (66% versus 46%).
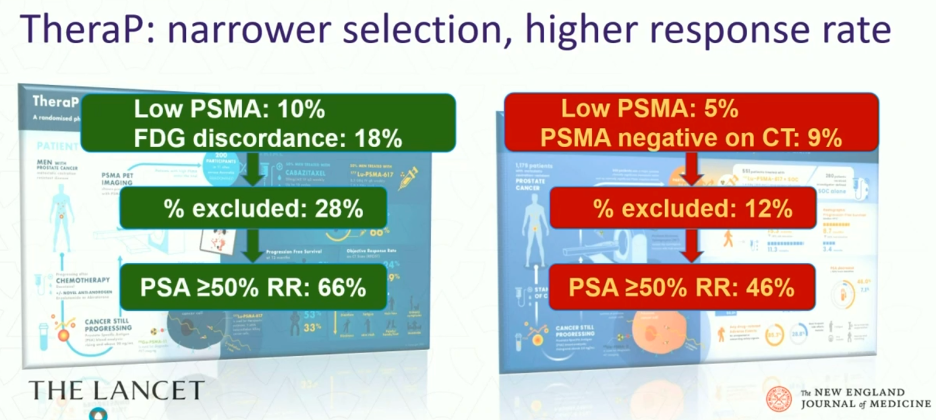
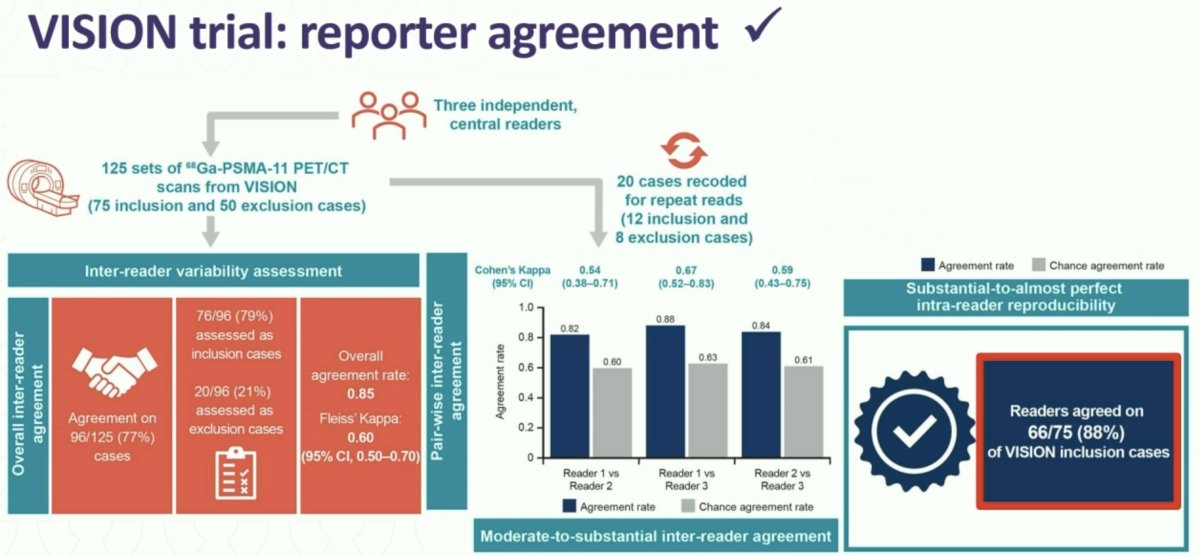
While the VISION selection criteria were less stringent compared to those of TheraP, one of the advantages of the VISION criteria is their ease of application and reproducibility, facilitating their application in clinical practice. A 2023 VISION substudy of 125 PET/CT scans randomly selected from VISION and retrospectively assessed by three independent central readers demonstrated that the overall agreement rate was 0.85 and all readers agreed on 66/75 VISION inclusion cases.4
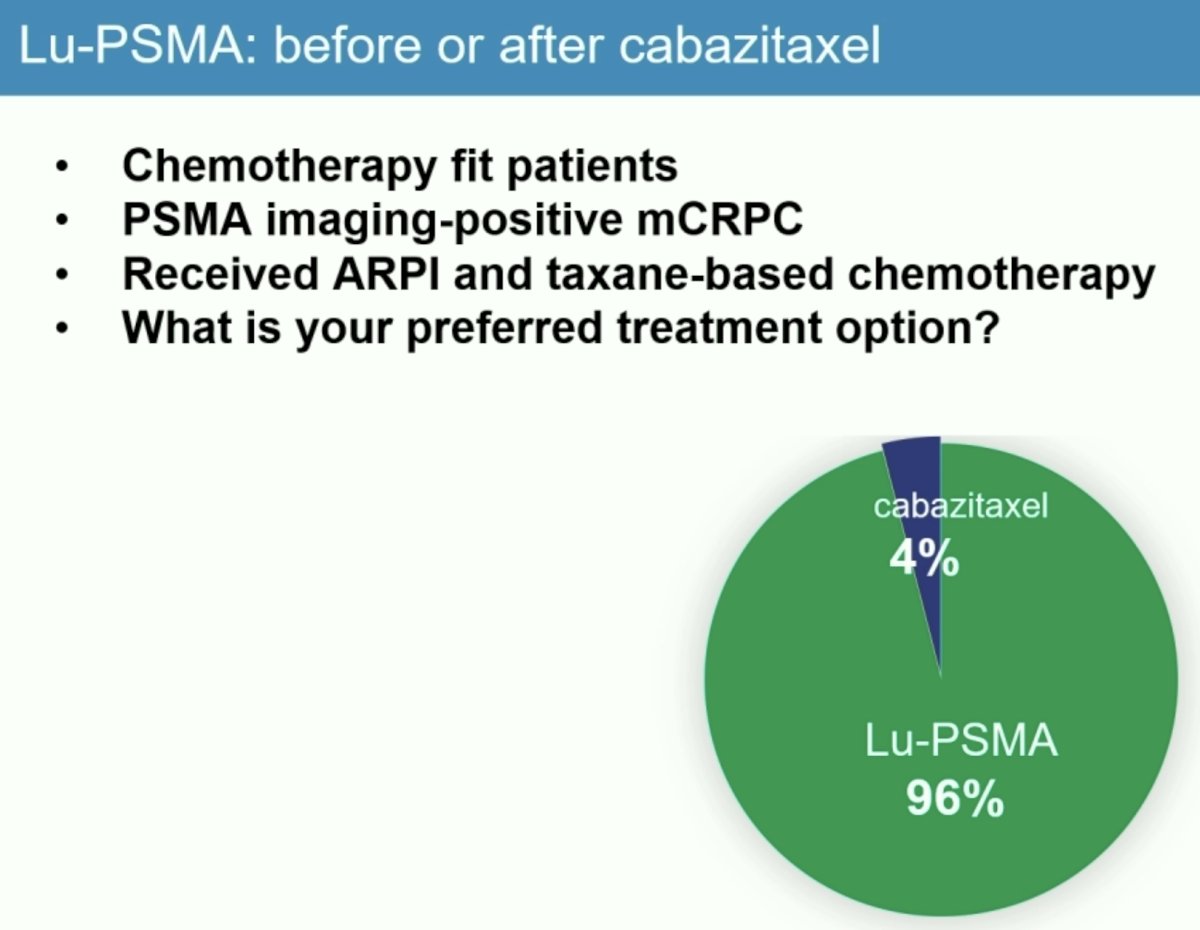
Dr. Hofman next presented unpublished consensus data from the most recent 2024 Advanced Prostate Cancer Consensus Conference (APCCC). In a survey of all expert attendees, 96% responded that Lu-PSMA was their favored treatment option for chemotherapy-fit patient, with PSMA-positive mCRPC, and who had received a prior ARPI and taxane-based chemotherapy.
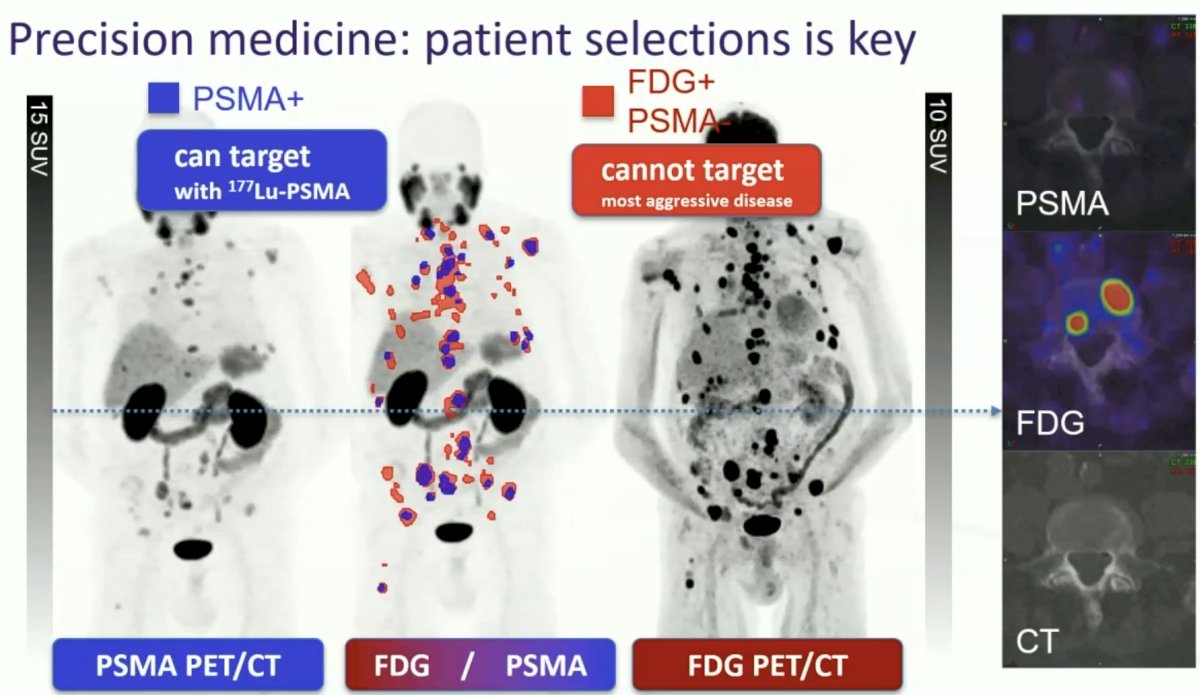
What about imaging requirements to determine patient eligibility for Lu-PSMA? 33% responded that PSMA PET alone was enough, and an additional 43% responded that additional FDG could be performed in equivocal cases. However, Dr. Hofman argued that patients should undergo both PSMA and FDG PET as some patients with PSMA PET-positive disease may have concurrent high-volume FDG PET-positive disease that may portend a poor response to Lu-PSMA. A case example is imaged below, whereby this patient has high volume FDG-positive disease (annotated in red) that may have been understaged had PSMA PET only had been performed. He argued that knowing the true disease extent allows us to make better decisions for our patients.
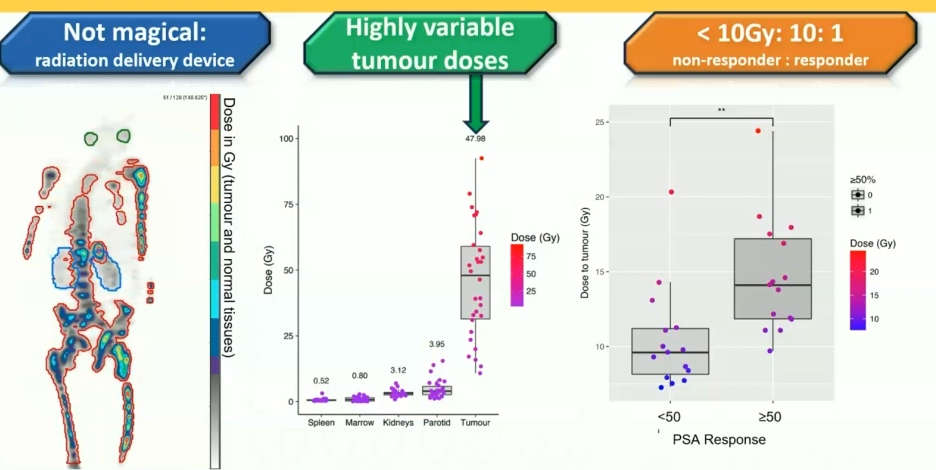
177Lu-PSMA-617 delivers high absorbed doses to tumor, with a significant correlation between whole-body tumor dose and PSA response. Patients receiving <10 Gy are unlikely to achieve a PSA50 response (ratio of responders to non-responders with dose <10 Gy is 1:10). There are highly variable tumor doses observed with higher doses, whereas doses to normal organs, such as the spleen, marrow, kidneys, and parotid glands remains low, irrespective of dose administered.5 This provides a rationale for a tumor burden-adapted dose escalation to enhance treatment efficacy.
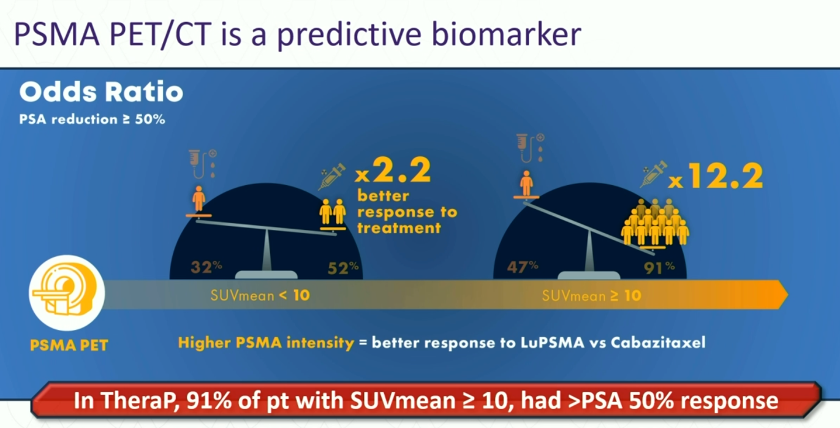
What parameters can we use to predict response to Lu-PSMA in mCRPC patients? Compared to cabazitaxel treatment, the odds of a PSA response to 177Lu-PSMA-617 were significantly higher for men with an SUVmean ≥10, compared to those with SUVmean <10 in TheraP (OR: 12.2 versus 2.2). High FDG-PET metabolic tumor volume was associated with lower responses regardless of treatment with cabazitaxel or 177Lu-PSMA-617, warranting further research for treatment intensification.6
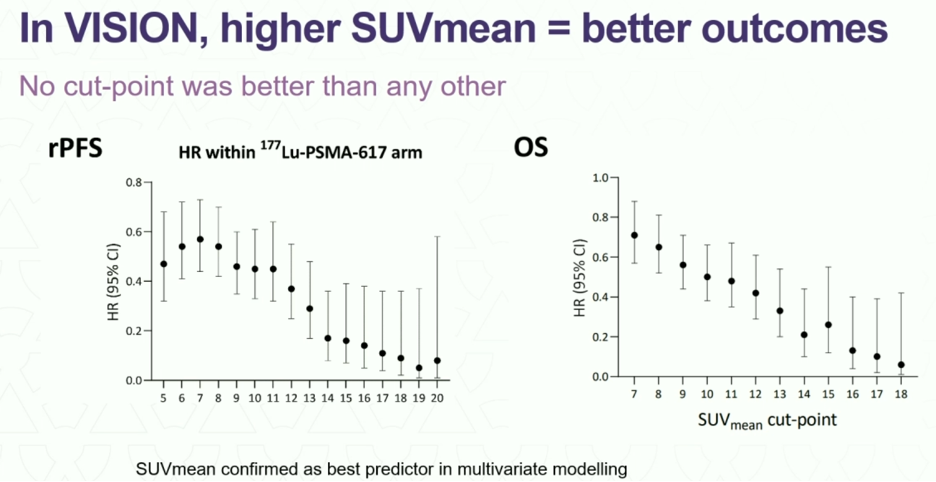
In VISION, no dichotomous analysis was performed; however, improved radiographic progression-free and overall survivals were observed for patients with higher SUVmeans.
Another technique to predict treatment response is to calculate the tumor-to-salivary gland uptake ratio. In 2023, Hotta et al. demonstrated that patients with higher ratios treated with LuPSMA have superior PSA progression-free and overall survival outcomes.7

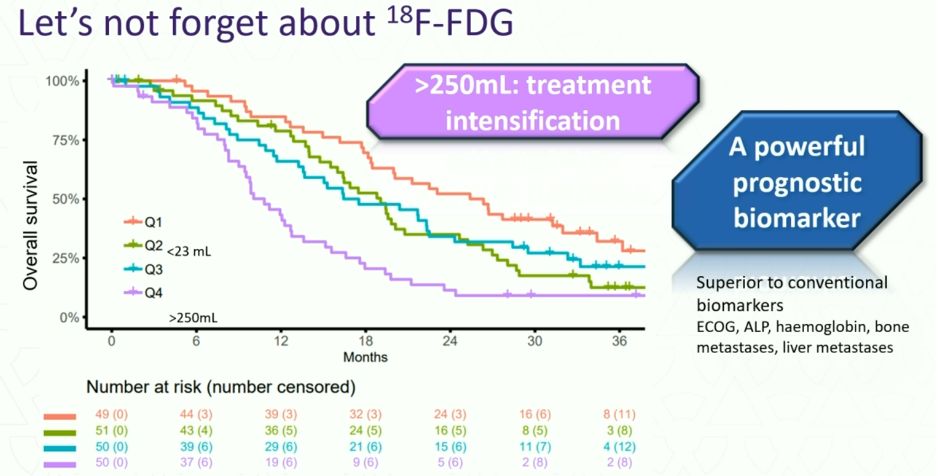
Dr. Hofman emphasized that we must not forget about 18F-FDG, which remains a powerful prognostic biomarker that outperforms conventional biomarkers such as ECOG, ALP, hemoglobin, bone metastases, and liver metastases for predicting survival outcomes.
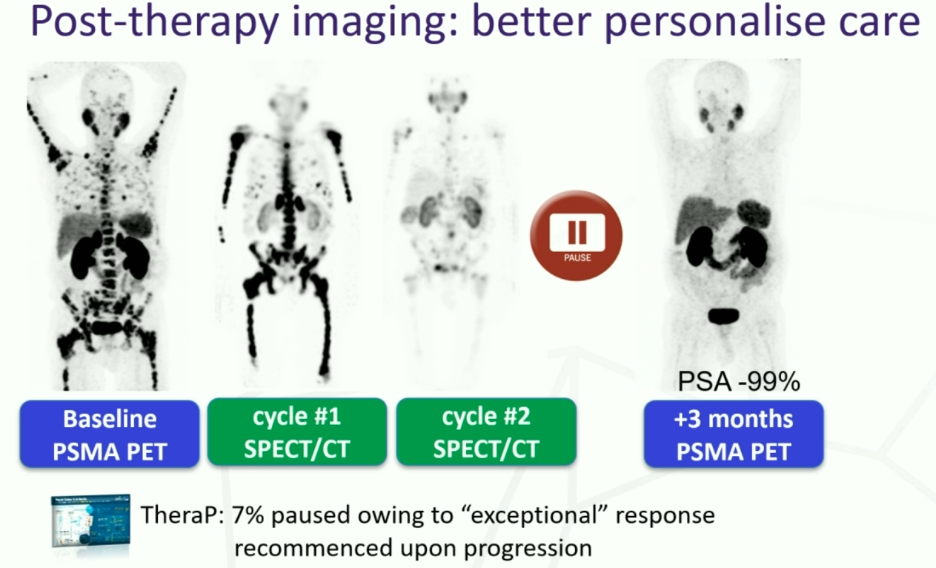
How many cycles of Lu-PSMA should eligible patients receive? In TheraP, a post-treatment SPECT/CT was performed and if patients had tracer uptake lower than that of the liver, then patients were considered for treatment suspension. Upon progression, patients underwent repeat staging with a PSMA/FDG PET and were considered for retreatment, if suitable. Conversely, in VISION, patients received four cycles and an additional two cycles if clinically benefiting.
How much 177Lu-PSMA should we administer? Should responding patients receive further consolidation as is typically performed with systemic therapy or should they be re-challenged when there is evidence of disease progression? Dr. Hofman argued that it should be the latter in most cases. As summarized in the graph below, this TheraP trial participant received 20 cycles of 177Lu-PSMA-617 with no dose-limiting toxicity and excellent responses to each Lu-PSMA re-challenge. Had this patient received more than six cycles in 2019, he likely would have had minimal benefits with regards to disease remission at the price of additional, likely unnecessary therapy.
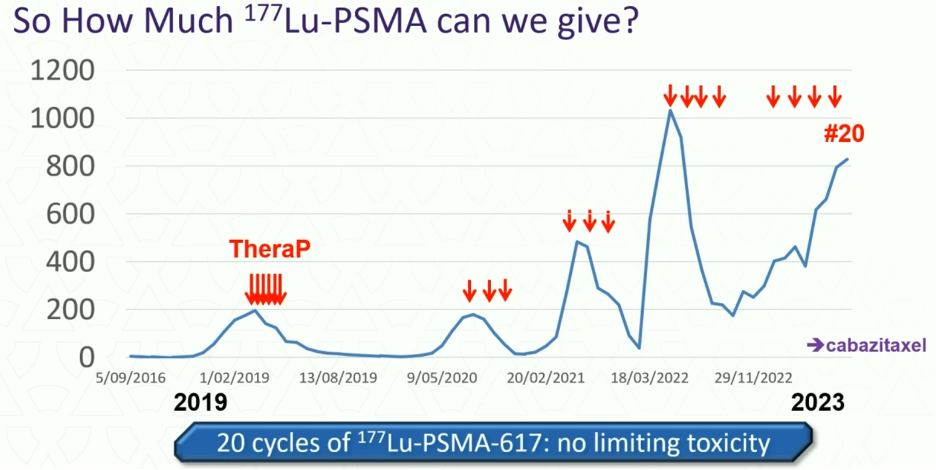
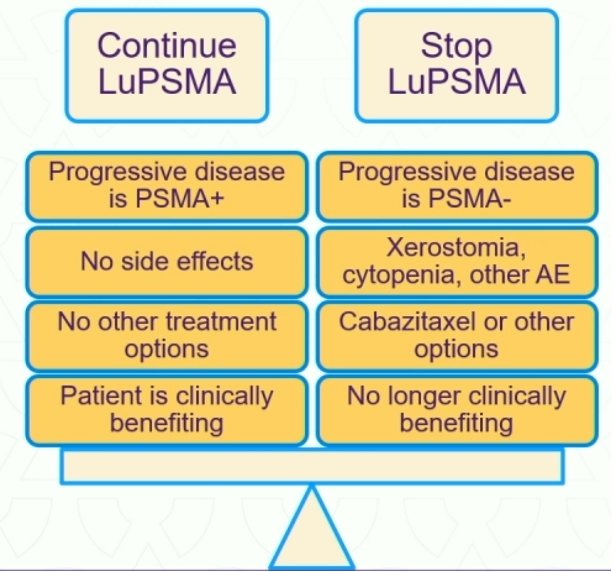
Conversely, when should LuPSMA therapy be stopped? If patients are no longer responding (PSA increase 25% above nadir, confirmed on repeat testing or progressive disease on imaging) and/or experiencing bothersome side effects, then LuPSMA therapy discontinuation should be considered.
Dr. Hofman concluded:
- Use LuPSMA post androgen receptor pathway inhibitor & taxane chemotherapy in mCRPC patients.
- It is reasonable to use LuPSMA prior to taxane chemotherapy if medically unsuitable.
- There is an increasing response rate with increasing PSMA intensity.
- Use FDG PET/CT to identify PSMA-negative sites of bone metastases.
- Close collaboration of nuclear medicine, medical oncology, urology, and radiation oncology is helpful for treating these patients.
- So many clinical trials are currently underway.
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024
References:
1. FDA approves Pluvicto for metastatic castration-resistant prostate cancer. Accessed on June 8, 2024.
2. Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
3. Swiha M, Papa N, Sabahi Z, et al. Development of a Visually Calculated SUVmean (HIT Score) on Screening PSMA PET/CT to Predict Treatment Response to 177Lu-PSMA Therapy: Comparison with Quantitative SUVmean and Patient Outcomes. J Nucl Med. 2024.
4. Kuo PH, Yoo DC, Avery R, et al. A VISION Substudy of Reader Agreement on 68Ga-PSMA-11 PET/CT Scan Interpretation to Determine Patient Eligibility for 177Lu-PSMA-617 Radioligand Therapy. J Nucl Med. 2023;64(8): 1259-65.
5. Violet J, Jackson P, Ferdinandus J, et al. Dosimetry of 177Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: Correlations Between Pretherapeutic Imaging and Whole-Body Tumor Dosimetry with Treatment Outcomes. J Nucl Med. 2019;60(4): 517-23.
6. Buteau JP, Martin AJ, Emmett L, et al. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [177Lu]Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): a biomarker analysis from a randomised, open-label, phase 2 trial. Lancet Oncol. 2022;23(11): 1389-97.
7. Hotta M, Gafita A, Murthy V, et al. PSMA PET Tumor-to-Salivary Gland Ratio to Predict Response to [177Lu]PSMA Radioligand Therapy: An International Multicenter Retrospective Study. J Nucl Med. 2023;64(7): 1024-9.


