(UroToday.com) The Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024 was host to a prostate cancer therapy session. Dr. Josef Zahner presented the first dosimetry results of a study evaluating the influence of androgen receptor pathway inhibitors (ARPIs) on absorbed doses in metastatic castrate-resistant prostate cancer (mCRPC) patients undergoing 177Lu-PSMA-617 therapy.
Preliminary data have suggested that ARPIs may enhance PSMA expression.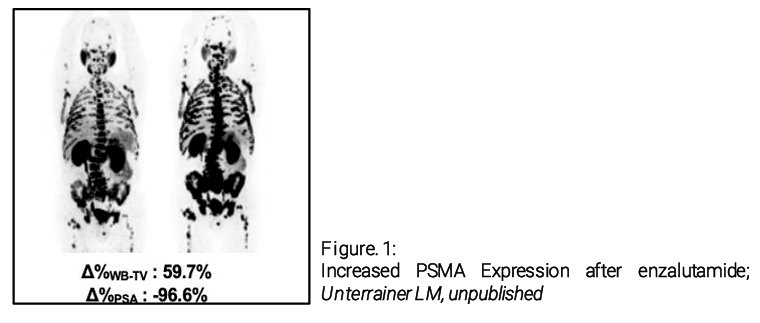
The recently published ENZA-p trial has demonstrated that combining 177Lu-PSMA radioligand therapy with enzalutamide improves progression-free survival in mCRPC patients.1 This may be secondary to potential:
- Synergistic effects: ARPIs acting as potential enhancer medications for PSMA radioligand therapy
- Additive effect: ‘PSMA flare phenomenon’ without therapeutic relevance
Does adding an ARPI to PSMA radioligand therapy increase the absorbed dose in tumor lesions?
This was a retrospective single-center analysis of mCRPC patients who underwent the 1st cycle of 177Lu-PSMA-617 or PSMA-I&T radioligand therapy, with or without concomitant ARPI therapy. Patients were matched into two groups (‘combination’ versus ‘control’ groups) by radioligand therapy received. These groups were comparable for total tumor volume.
Quantitative abdominal SPECT images were acquired at 24 (+CT), 48, and 74 hours after administration of the first cycle of 177Lu-PSMA radioligand therapy. Dosimetry was performed using MIM SurePlan™ MRT, MIMSoftware Inc. Tumor lesions were segmented on the SPECT at 24 hours. The organs at risk (kidney, spleen, liver) were delineated on the corresponding CT. Absorbed dose calculations were based on a mono-exponential curve fit and included local density scaling.
This analysis included 50 patients, of whom 37 and 13 received PSMA-617 and PSMA-I&T, respectively. In the PSMA-617 group 109 tumor lesions were included, of which 55 and 54 were in the combination and control groups, respectively. In the PSMA-I&T group, 39 tumor lesions were included. As demonstrated in the bar graphs below, there was no significant difference in the mean tumor lesion absorbed doses between patients in the combination and control groups.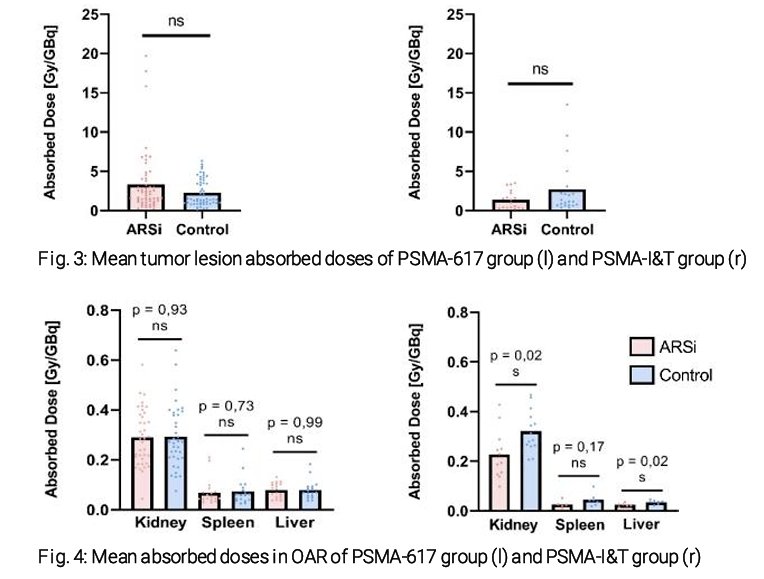
In the PSMA-617-treated cohort, the mean tumoral lesion absorbed doses were 3.35 Gy/GBq in the combination group, compared to 2.28 in the control group (p=0.07). Conversely in the PSMA-I&T-treated patients, the corresponding doses were 1.38 and 2.71 Gy/GBq, respectively (p=0.13).
Dr. Zahner concluded that:
- There were no significant differences in tumoral lesions absorbed doses between patients receiving combination radioligand + ARPI therapy, versus radioligand therapy alone.
- This suggests that the combination of radioligand + ARPI therapy most likely has an additive effect.
- PSMA overexpression by an APRI is likely a ‘PSMA flare phenomenon’
Presented by: Josef Zahner, PhD, Department of Nuclear Medicine, LMU University Hospital, LMU Munich, Munich, Germany
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024
Reference:


