(UroToday.com) The Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024 was host to a prostate cancer therapy session. Dr. Oliver Sartor presented the updated results of PSMAfore, a phase 3 trial of [177Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (mCRPC).
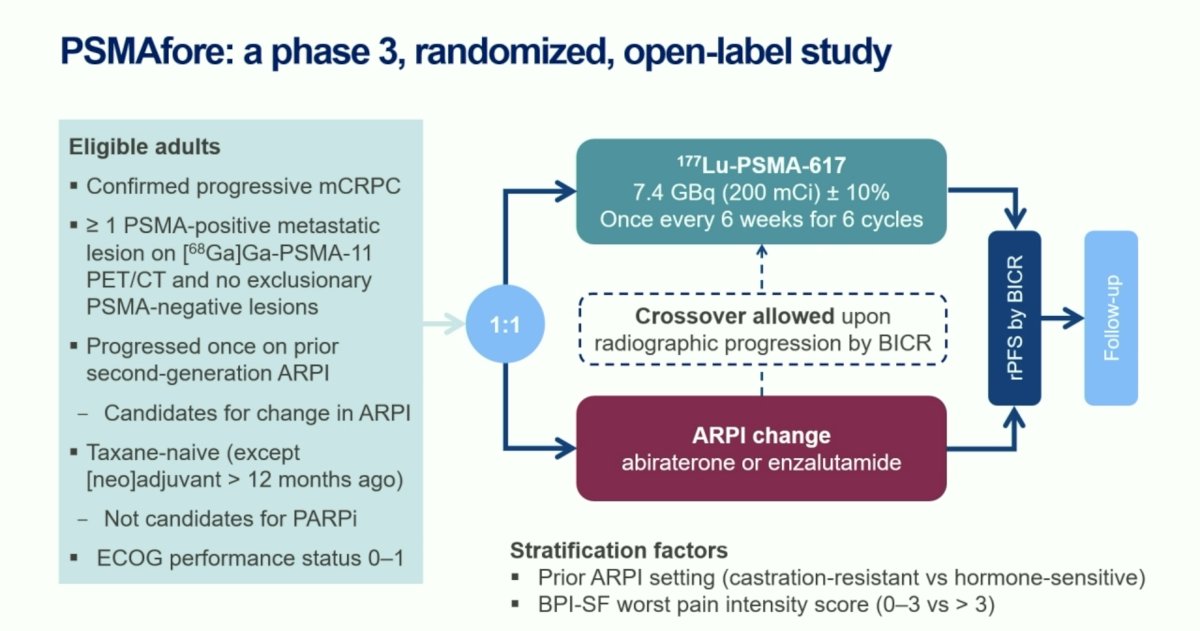
PSMAfore is a phase III trial that randomized mCRPC patients with ≥1 PMSA positive lesion and no exclusionary PSMA negative lesions by 68Ga-PSMA-11 PET/CT 1:1 to open-label 177Lu-PSMA-617 (7.4 GBq every 6 weeks for 6 cycles) or androgen receptor pathway inhibitor (ARPI) change (abiraterone or enzalutamide). Significantly, eligible patients could not be candidates for PARP inhibitor therapy and were required to be taxane-naïve or received taxane chemotherapy in the neoadjuvant or adjuvant setting >12 months ago. Patients randomized to ARPI could crossover to 177Lu-PSMA-617 following centrally reviewed radiographic progression. The study design is as follows:
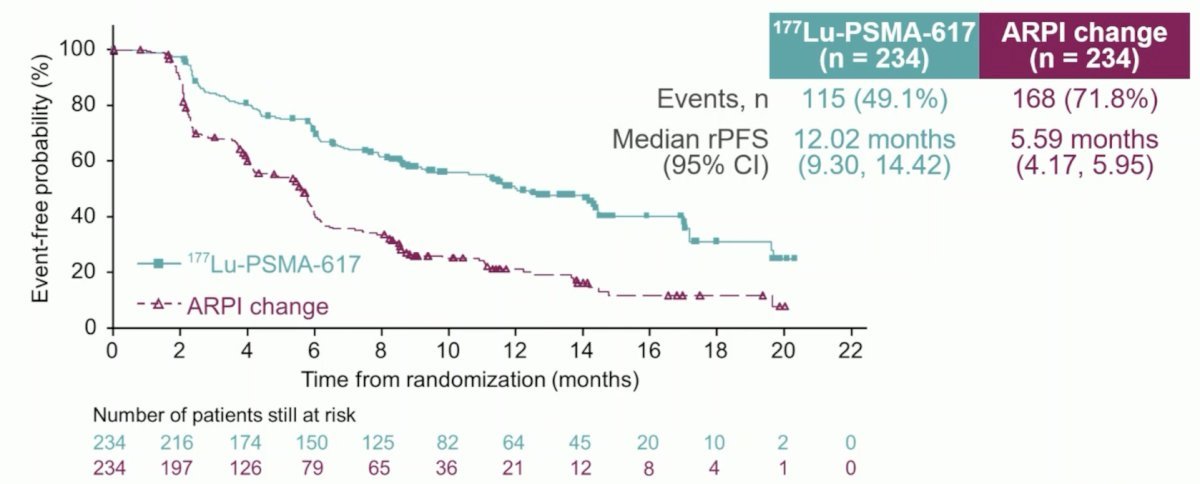
At the primary analysis (median follow-up, 7.3 months), the primary endpoint of radiographic progression-free survival was met (HR: 0.41, 95% CI: 0.29–0.56), which was similar to the results at the second interim analysis (HR: 0.43, 95% CI 0.33–0.54).
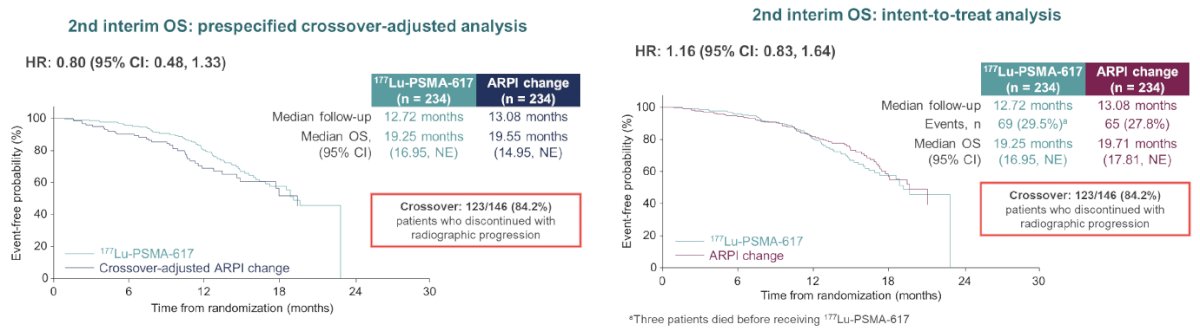
In the unadjusted, intention to treat analysis for overall survival, the hazard ratio was 1.16 (95% CI: 0.83–1.64). Of note, 84.2% of eligible patients in the ARPI control arm crossed over to 177Lu-PSMA-617 following radiographic progression.
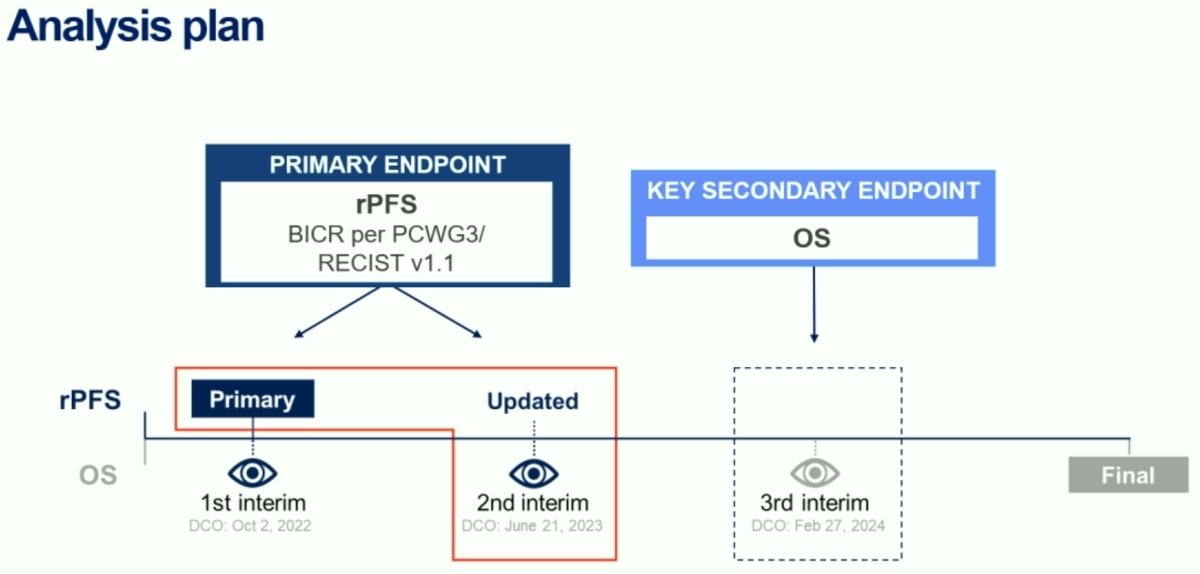
In this presentation, Dr. Sartor presented the results of the 3rd interim analysis (data cut-off date: February 27, 2024), including an update of the key secondary endpoint, overall survival.
The two treatment arms were well-balanced for baseline patient characteristics. The median patient age was 71–72 years. The median serum PSA level at study entry was 14.9–18.4 ng/ml. The most common site of metastatic disease was the bone (~87%). The most frequently used prior ARPI was abiraterone (50.9–55.6%).

Dr. Sartor re-presented the prior rPFS data from the 2nd interim analysis that demonstrated that 177Lu-PSMA-617 was associated with a significant rPFS improvement (HR: 0.43, 95% CI: 0.33–0.54). The objective response rate favored the 177Lu-PSMA-617 arm (51% versus 15%), with a complete radiologic response observed in 21.1% of 177Lu-PSMA-617-treated patients (2.7% in ARPI change arm).
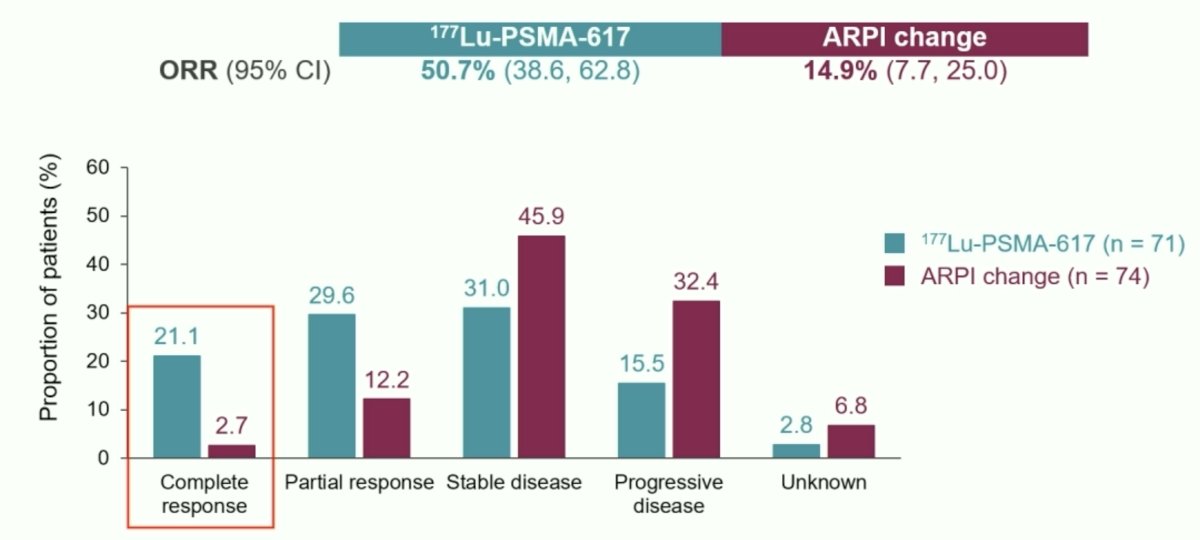
A PSA50 response rate was observed in 58% of 177Lu-PSMA-617-treated patients, versus 20.4% of patients in the ARPI switch arm.

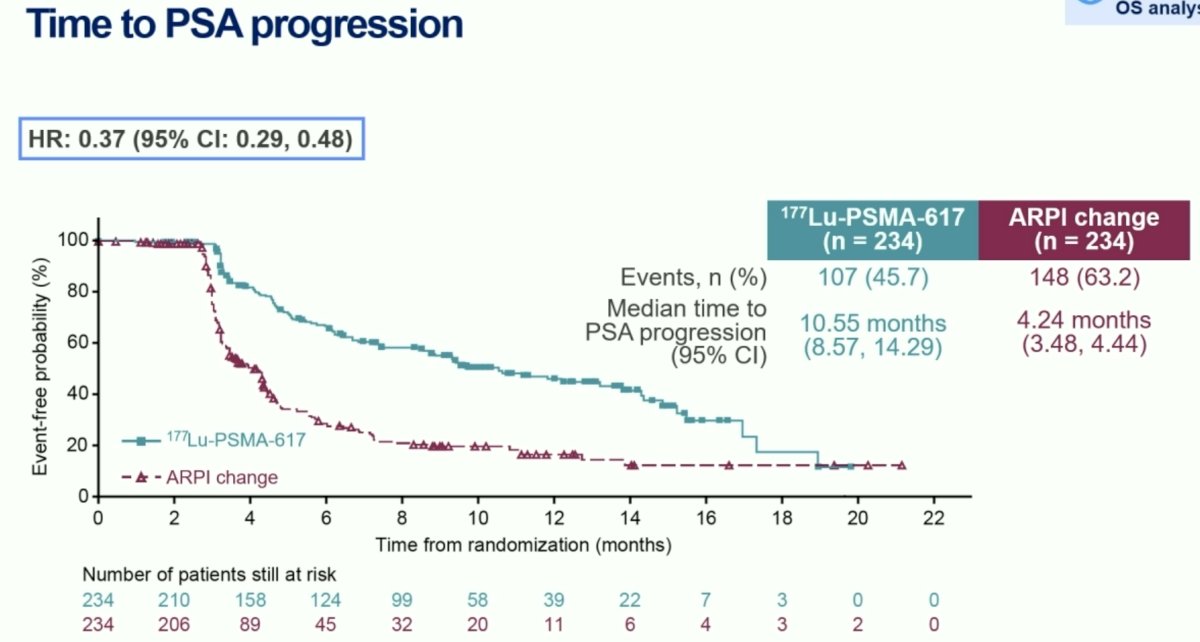
Time to PSA progression also significantly favored the 177Lu-PSMA-617 arm (HR: 0.37, 95% CI: 0.29–0.48):
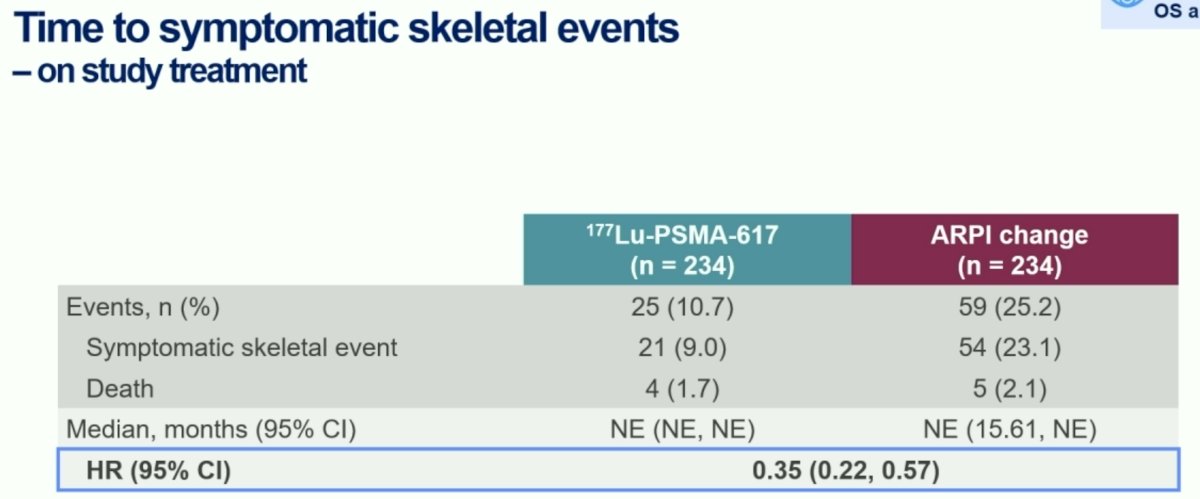
Time to symptomatic skeletal events, a particularly meaningful endpoint for mCRPC patients, strongly favored the 177Lu-PSMA-617 arm (HR: 0.35, 95% CI: 0.22–0.57).
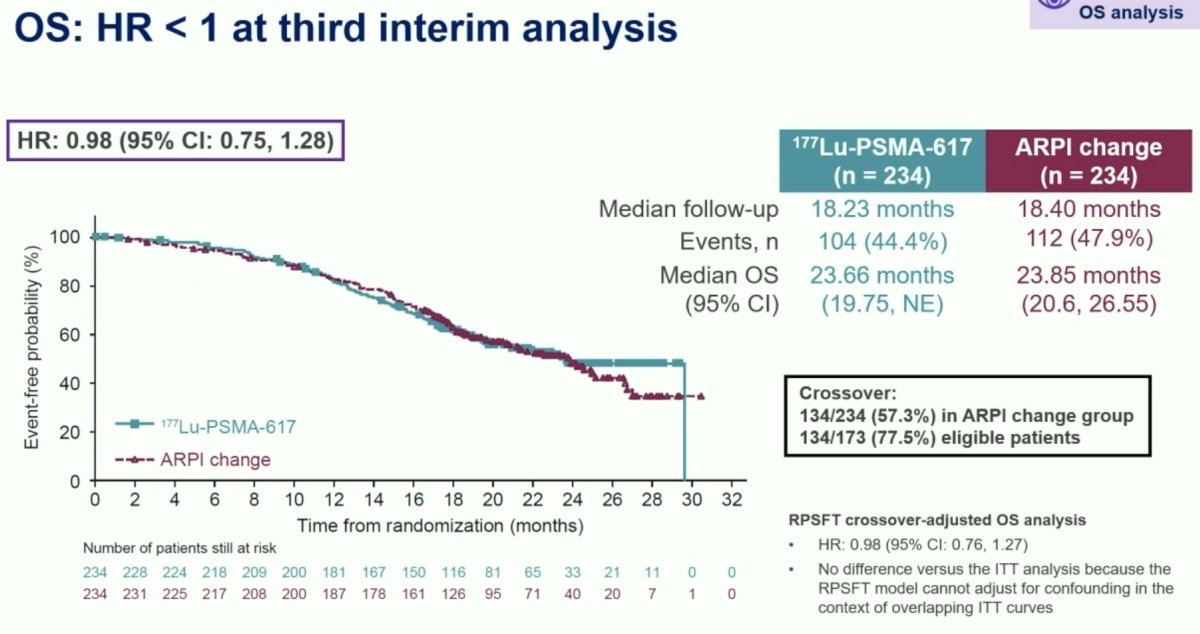
With regards to overall survival, this third interim analysis now demonstrates that the hazard ratio is <1, with a value of 0.98 (95% CI: 0.75–1.28). To date, there have been 104 deaths in the 177Lu-PSMA-617 arm and 112 in the ARPI change arm, reflecting a data maturity of 44.4–47.9%. The median overall survival was 23.7 months and 23.9 months in the two arms, respectively. Of note, 77.5% of all eligible patients in the ARPI change group crossed over to 177Lu-PSMA-617 treatment.
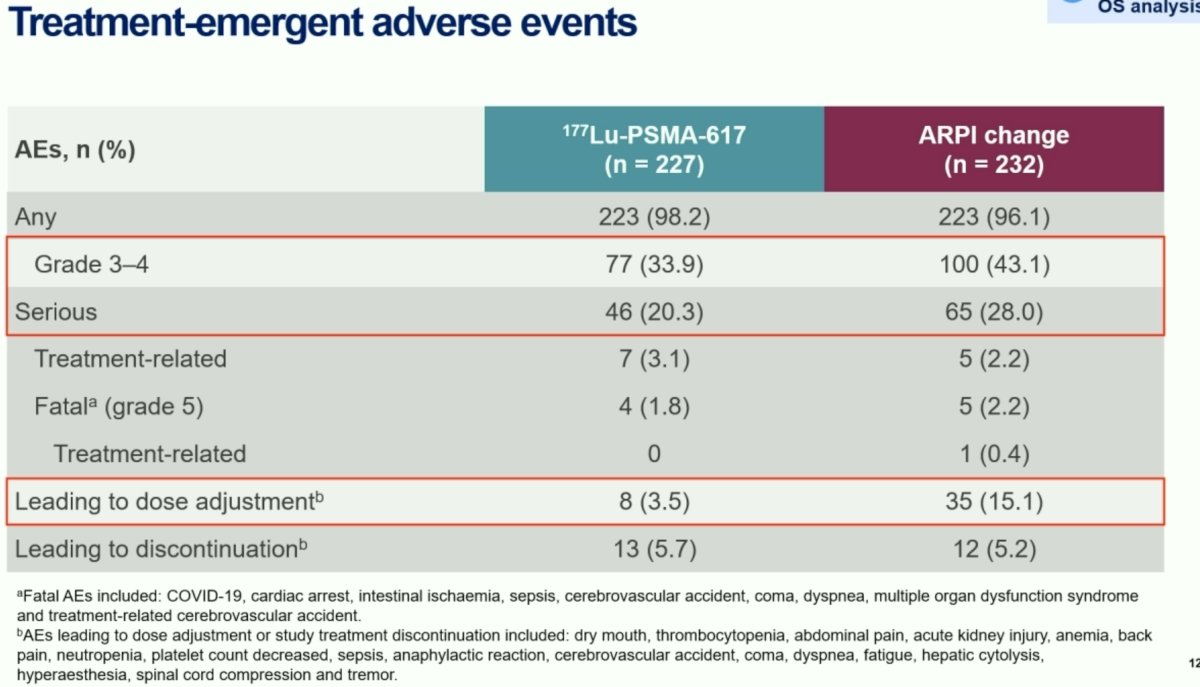
With regards to adverse events, grade 3-4 events were less frequent in the Lu-PSMA arm (34% versus 43%). Similarly, serious adverse events were also less common with 177Lu-PSMA-617 treatment (20% versus 28%). Adverse events leading to dose adjustment occurred less commonly with 177Lu-PSMA-617 treatment (3.5% versus 15%).
Dr. Sartor concluded as follows:
- 177Lu-PSMA-617 treatment prolonged rPFS versus ARPI change in taxane-naïve mCRPC patients.
- Secondary and exploratory endpoints also favored 177Lu-PSMA-617 treatment.
- There was no difference in overall survival with 177Lu-PSMA-617 versus ARPI change, but results are likely confounded by substantial rate of crossover.
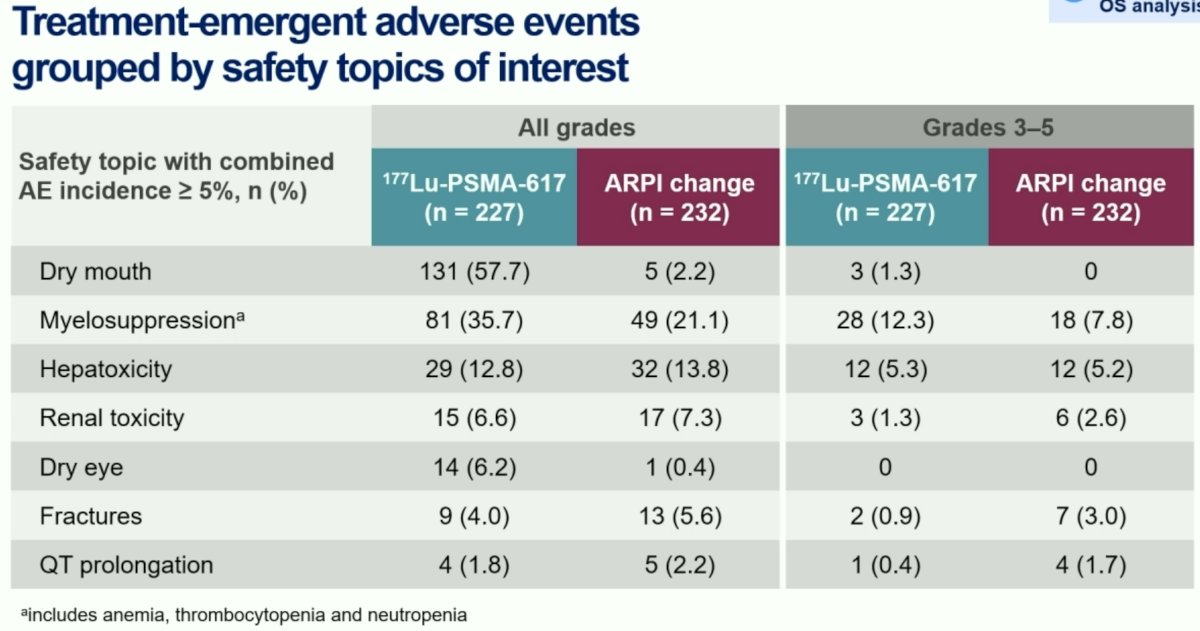
- 177Lu-PSMA-617 had a favorable safety profile and was well-tolerated.
Presented by: Oliver Sartor, MD, Professor, Department of Medical Oncology, Mayo Clinic, Rochester, MN
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024


