e(UroToday.com) The Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024 was host to a prostate cancer therapy session. Vishnu Murthy presented the results of a US expanded access program evaluating the efficacy and toxicity of 177Lu-PSMA-617 for metastatic castration-resistant prostate cancer (mCRPC) in a real-world setting and compared these results to those from the phase 3 VISION trial.
VISION was an international, open-label, phase 3 trial that evaluated 177Lu-PSMA-617 in mCRPC patients previously treated with an androgen receptor pathway inhibitor (ARPI) and 1-2 taxane regimens and who had PSMA-positive 68Ga-PSMA-PET/CT scans. Between June 2018 and October 2019, 831 patients were randomly assigned in a 2:1 ratio to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks for four to six cycles) plus protocol-permitted standard care or standard care alone. At a median follow-up of 20.9 months, 177Lu-PSMA-617 plus standard care significantly prolonged, as compared with standard of care, both radiographic progression-free survival (rPFS; median: 8.7 versus 3.4 months; HR: 0.40, p<0.001) and overall survival (median: 15.3 versus 11.3 months; HR: 0.62; 95% CI: 0.52 to 0.74, p<0.001).1
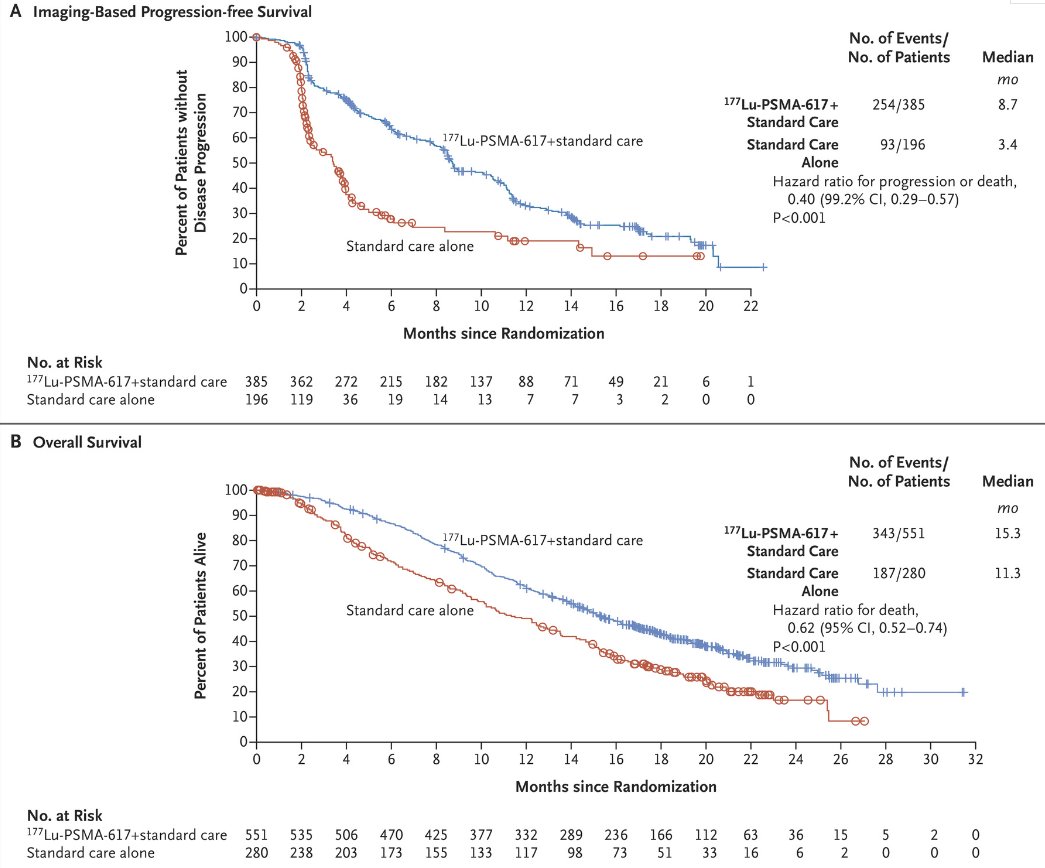
Based on these results, along with those from the TheraP trial, 2 177Lu-PSMA-617 (PLUVICTO®) was approved for the treatment of adult mCRPC patients who experience disease progression following treatment with an ARPI and taxane-based chemotherapy.3 The expanded access program of 177Lu-PSMA-617 (NCT04825652) was opened to provide access to 177Lu-PSMA-617 for eligible patients until regulatory approval was obtained. This study aimed to evaluate the efficacy and safety profile of 177Lu- PSMA-617 within the expanded access program and compare the results with those from the VISION trial.
Patients enrolled in the expanded access program at four institutions across the United States (UCLA, UCSF, Tulane, Johns Hopkins) with available toxicity and outcome data were included. Patients underwent a baseline 68Ga-PSMA-11 or 18F-DCFPyL PSMA PET/CT scan that was locally interpreted by nuclear medicine physicians according to VISION PET criteria to define treatment eligibility. Eligible patients received 7.4 GBq (200 mCi) +/- 10% of 177Lu-PSMA-617 once every six weeks for up to six cycles, and treatment cycles continued until disease progression, severe toxicity, or patient withdrawal. Laboratory tests (comprehensive metabolic panel, estimated GFR, complete blood count, and PSA) were performed within four weeks of
each cycle and 4-6 weeks after the last cycle. Patients were retrospectively screened for incidences of hematologic toxicity using Common Terminology Criteria for
Adverse Events (CTCAE) v5.0. Efficacy outcome measures included ≥50% PSA decline response rates (PSA-RR) and overall survival. Baseline characteristics, efficacy, and toxicity data from the VISION trial were extracted from the publication, and differences between the expanded access program and VISION were evaluated using t-tests of proportions.
A total of 117 patients from the real-world expanded access program were included. The baseline patient characteristics are summarized below. Compared to patients from the VISION trial, those in the expanded access program were significantly more likely to have received >1 prior ARPI (70% versus 46%) and have worse ECOG performance status (≥2: 195 versus 7%). Expanded access program patients were more likely to have nodal disease on baseline PSMA-PET/CT (61% versus 50%; p=0.031), while a similar proportion of expanded access program patients had lung (p=0.90), liver (p=0.35), and bone (p=0.76) disease on baseline PSMA-PET/CT, compared with VISION patients.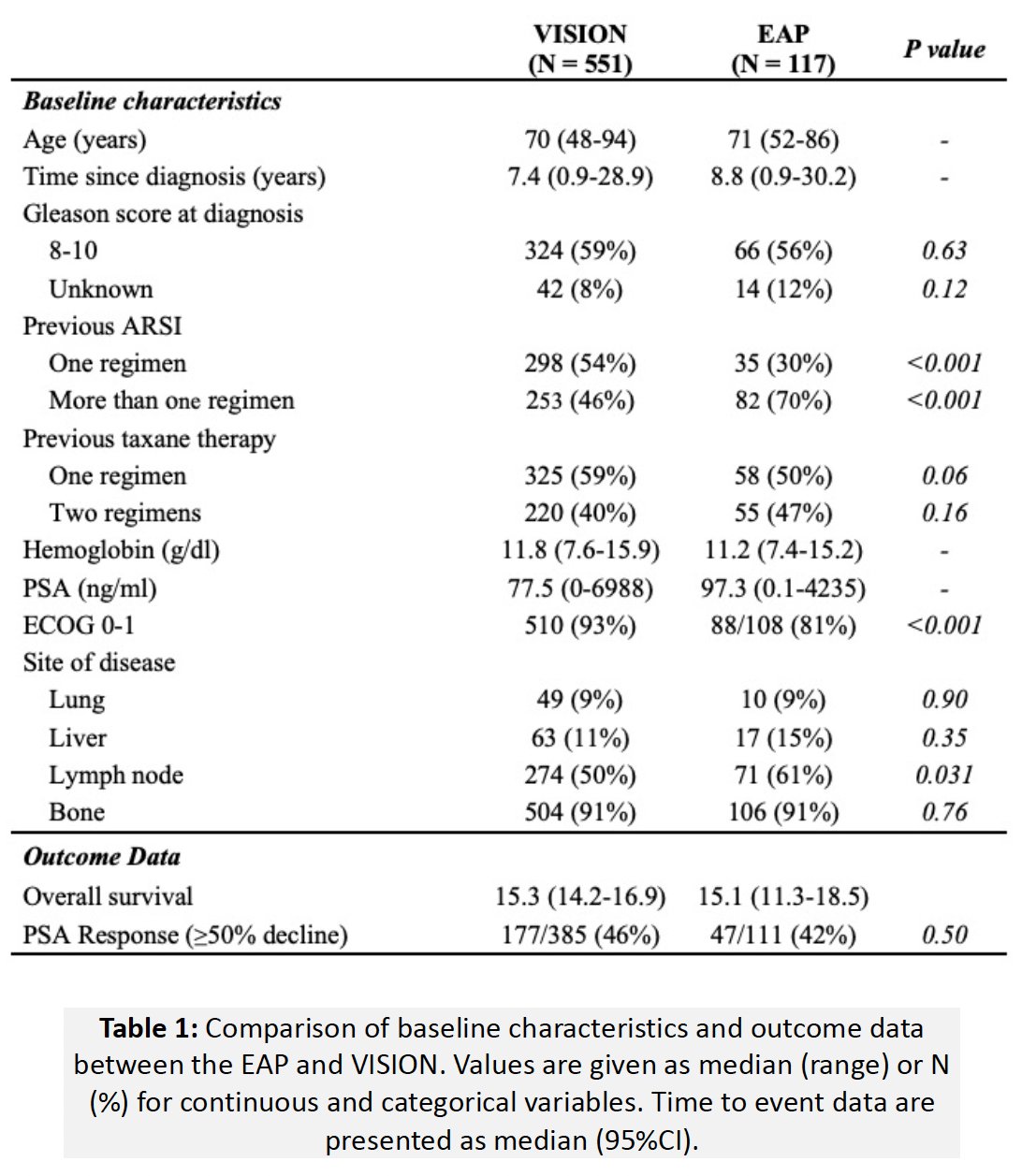
The median overall survival in this cohort was 15.1 months, which was nearly identical to that observed in the VISION cohort (15.3 months). The PSA50-RR was also similar in the two cohorts (42% versus 46%, p=0.50).
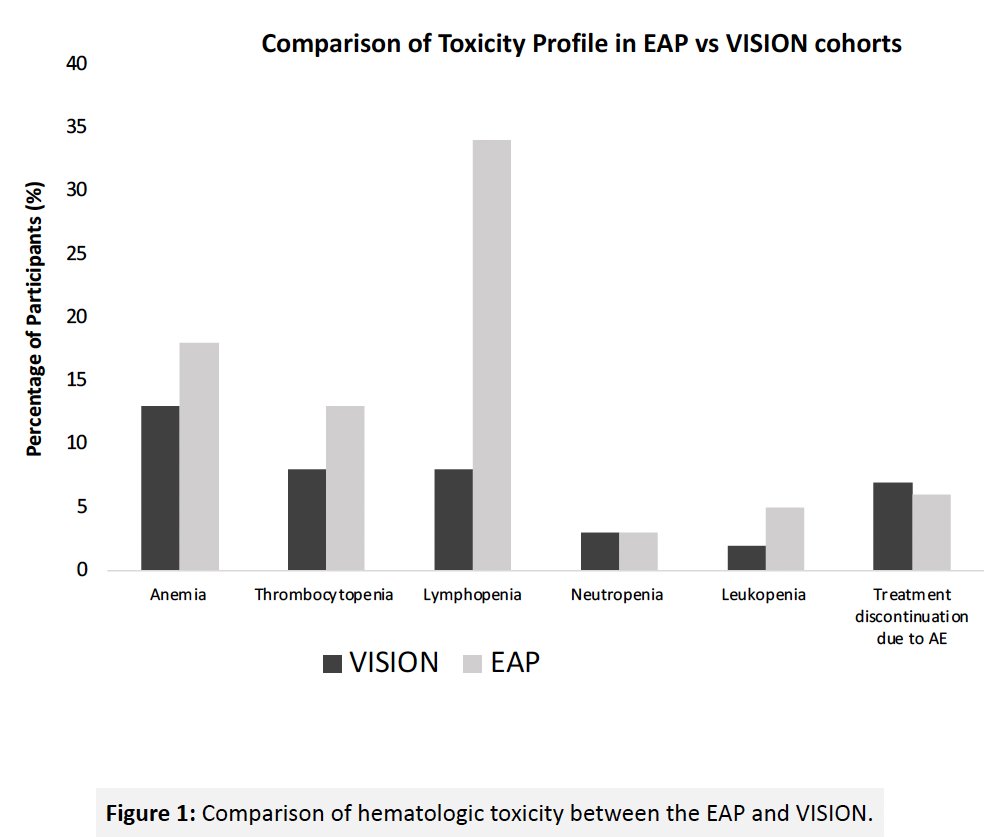
With regards to any grade adverse events, patients in the real-world cohort had a significantly higher incidence of:
- Lymphopenia (66% versus 14%, p<0.001)
- Neutropenia (32% versus 9%, p<0.001)
- Thrombocytopenia (49% versus 17%, p<0.001)
- Leukopenia (47% versus 12%, p<0.001)
- Anemia (42% versus 32%, p=0.04).
With regards to grade ≥3 adverse events, patients in the real-world cohort had a significantly higher incidence of grade ≥3 lymphopenia (34% vs 8%, p<0.001). A similar proportion of patients discontinued treatment due to any grade toxicity (13% vs 12%; p=0.78) within the expanded access program, compared with VISION patients.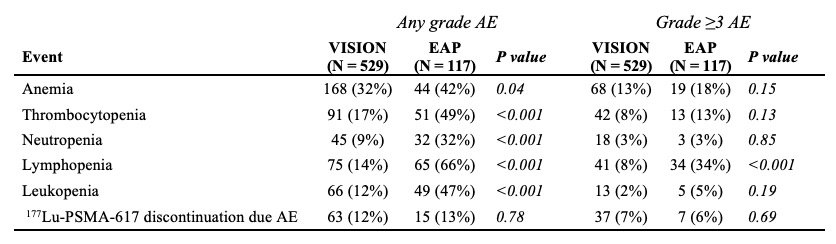
The study investigators concluded that patients with PSMA-positive mCRPC who received 177Lu-PSMA-617 within the expanded access program were later in their disease trajectory, achieved similar PSA-RR and overall survival, and had a similar safety profile compared with VISION trial patients.
Presented by: Vishnu Murthy, MD Candidate, David Geffen School of Medicine at UCLA, Los Angeles, CA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024
References:
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385:1901-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- FDA approves Pluvicto for metastatic castration-resistant prostate cancer. Accessed on June 8, 2024.


