(UroToday.com) The Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024 was host to a prostate cancer therapy session. Dr. Fuad Novruzov presented the results of a prospective phase 3 randomized study from Azerbaijan evaluating 225Ac-PSMA + 177Lu-PSMA tandem therapy for metastatic castration-resistant prostate cancer (mCRPC).
- While 225Ac-PSMA has demonstrated promising therapeutic efficacy in heavily pre-treated mCRPC patients, there are still some concerns regarding its safety profile and toxicity for organs at risk.1 Combining the alpha emitter 225Ac with the beta emitter 177Lu as tandem therapy at reduced doses may reduce the adverse effects of alpha emitters, while potentially increasing the therapeutic efficacy of beta emitters alone. The objective of this study was to present preliminary findings from a phase 3, single-center, prospective, randomized, two-arm controlled study evaluating 225Ac-PSMA + 177Lu-PSMA tandem therapy for mCRPC patients.
- Patients with androgen receptor pathway inhibitor (ARPI) and docetaxel pre-treated mCRPC who exhibited high uptake on PSMA PET were randomized to 225-Actinium / 177- Lutetium labeled PSMA (PSMA tandem treatment) versus the current standard-of-care with docetaxel. The study inclusion criteria were as follows:

- The primary study endpoints were progression-free survival and overall survival. Serial prostate-specific antigen (PSA) measurements were obtained for response assessment. Hematological and non-hematological adverse effects were recorded.
- Fifty patients were included in the PSMA treatment arm. Nearly all patients had evidence of lymph node (90%) and bone metastases (94%), and 52% had visceral metastases.
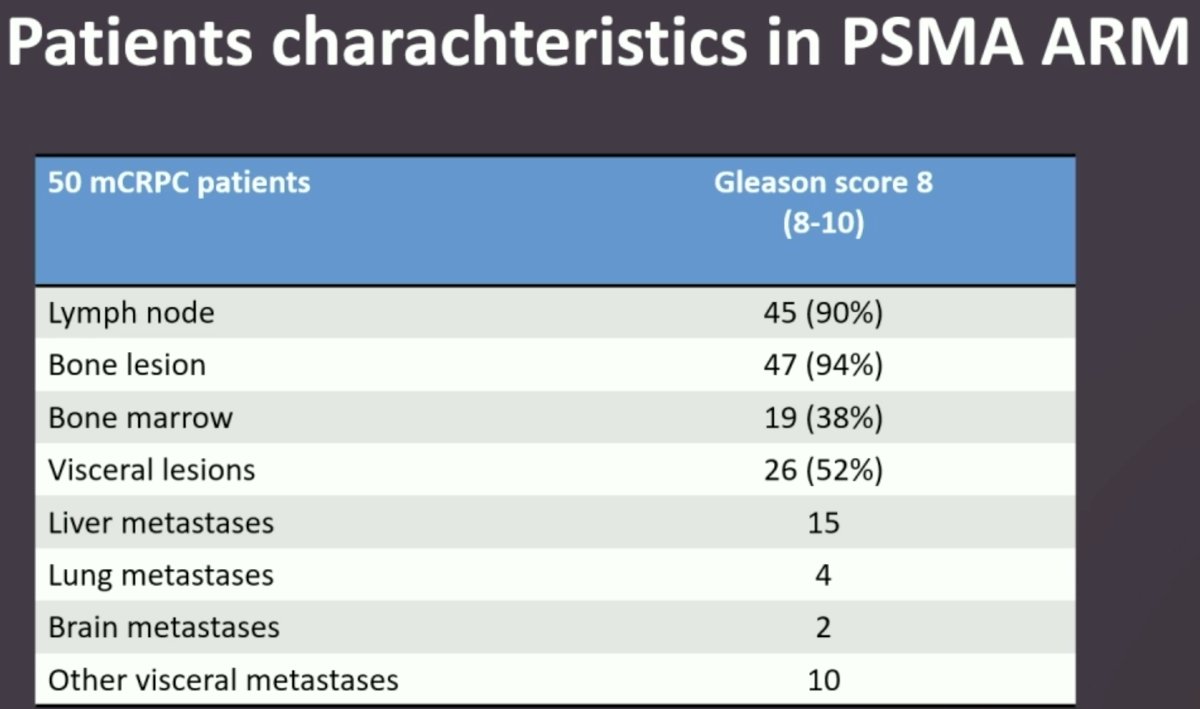
Among the 50 patients in the PSMA treatment arm, 32 (64%) received tandem therapy, 11 (22%) received only 225Ac-PSMA, and 7 (14%) received only 177Lu-PSMA.

A total of 93 patients were included in the study. The baseline characteristics by treatment arm are summarized below. The mean PSA at study entry was significantly higher in the PSMA arm (420.8 versus 280 ng/ml).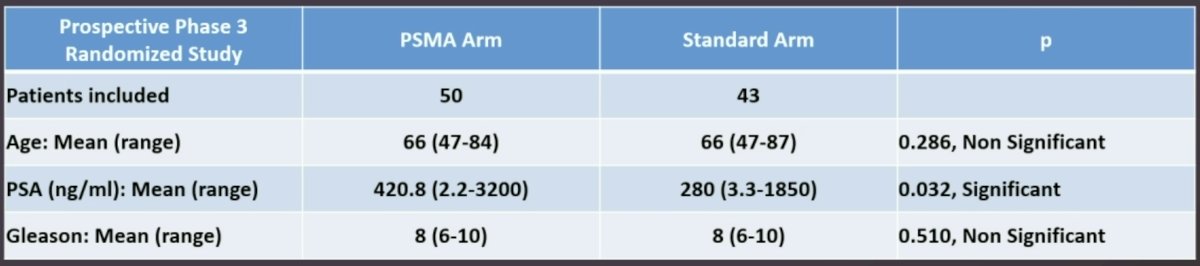
In the PSMA treatment arm, no severe hematologic toxicity or nephrotoxicity was observed. The most common toxicity reported was grade 1-2 xerostomia, observed in 36% of patients (xerostomia by radioligand therapy received is summarized below). In the standard arm, treatment discontinuation due to adverse effects/toxicity affected 10% of patients.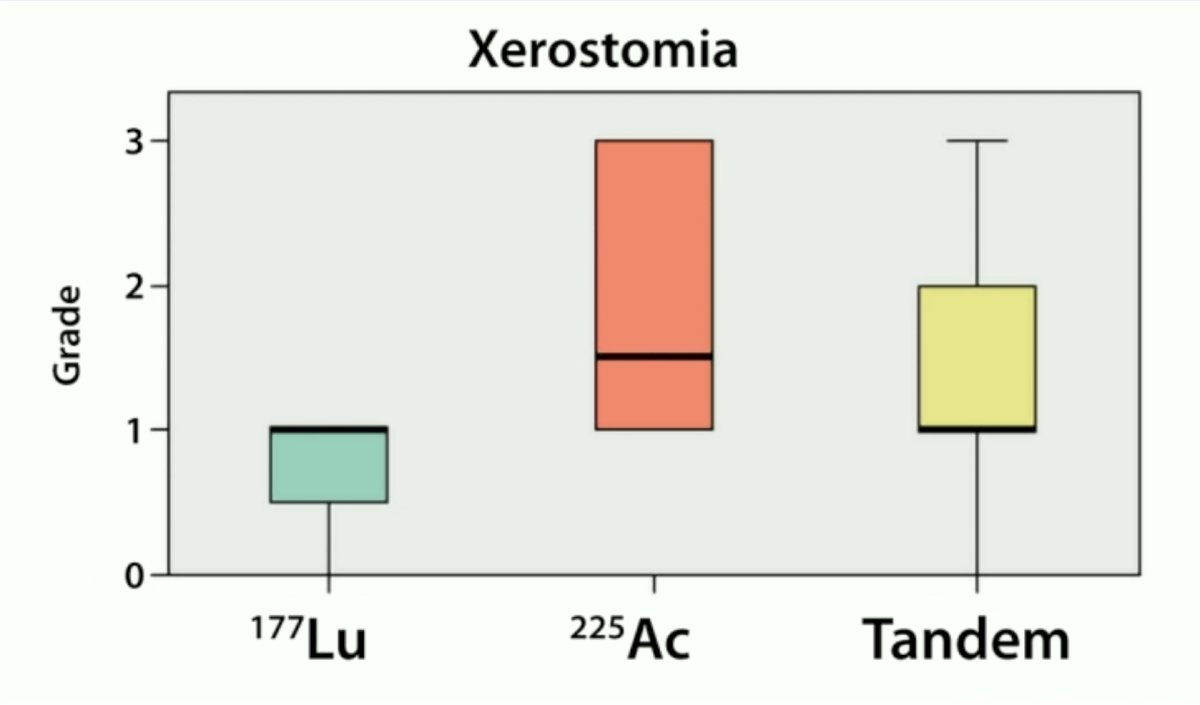
The median progression-free survival was 3.8 months in the PSMA arm versus 1.1 months in the standard arm (p<0.001). The progression-free survival rates at 100 days were 53% and 9%, respectively (p<0.001). 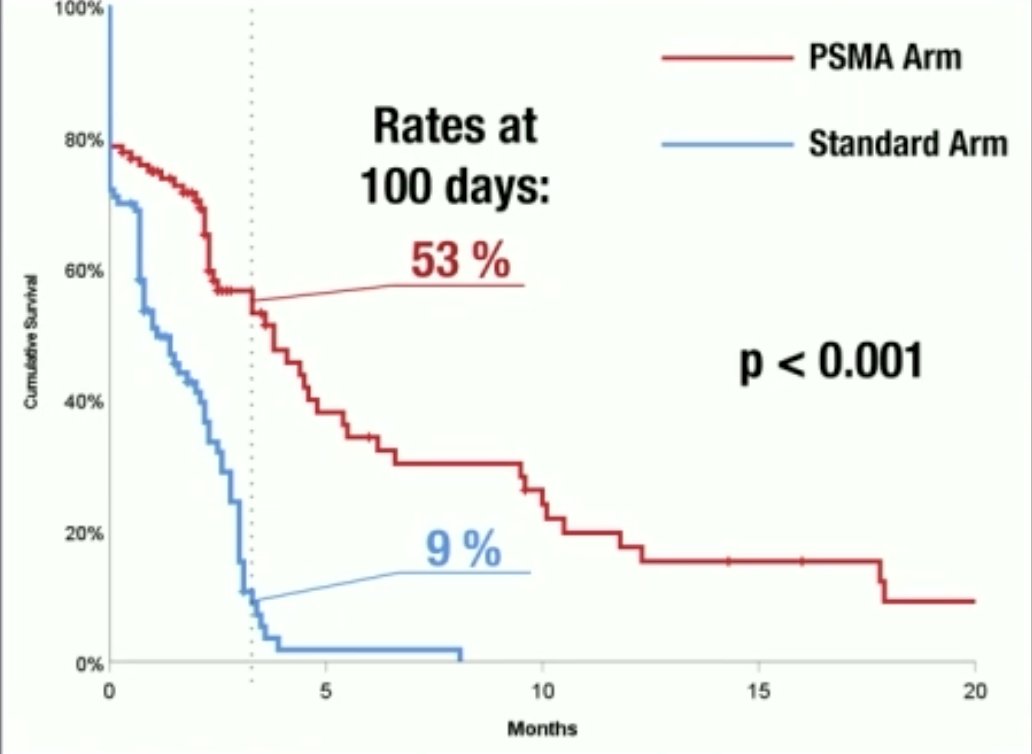
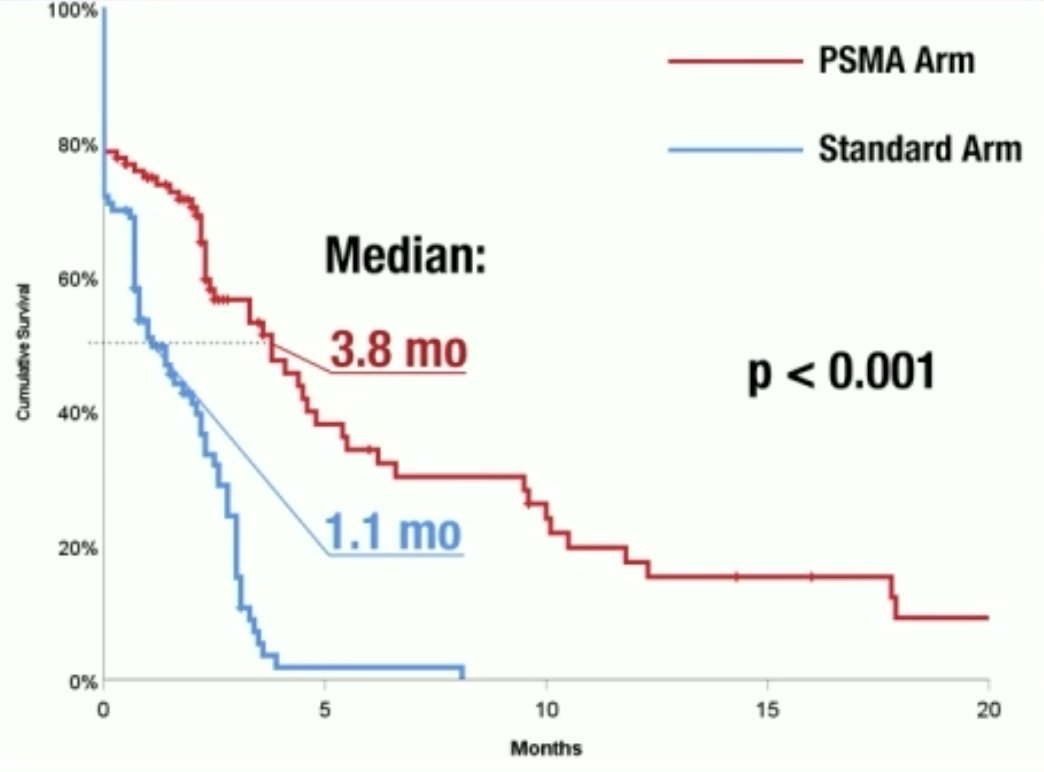
There were no significant differences in overall survival (PSMA arm: 19.5 months; Standard arm: 20 months).
PSA50 responses were observed more frequently in the PSMA arm (36% versus 28%, p=0.037).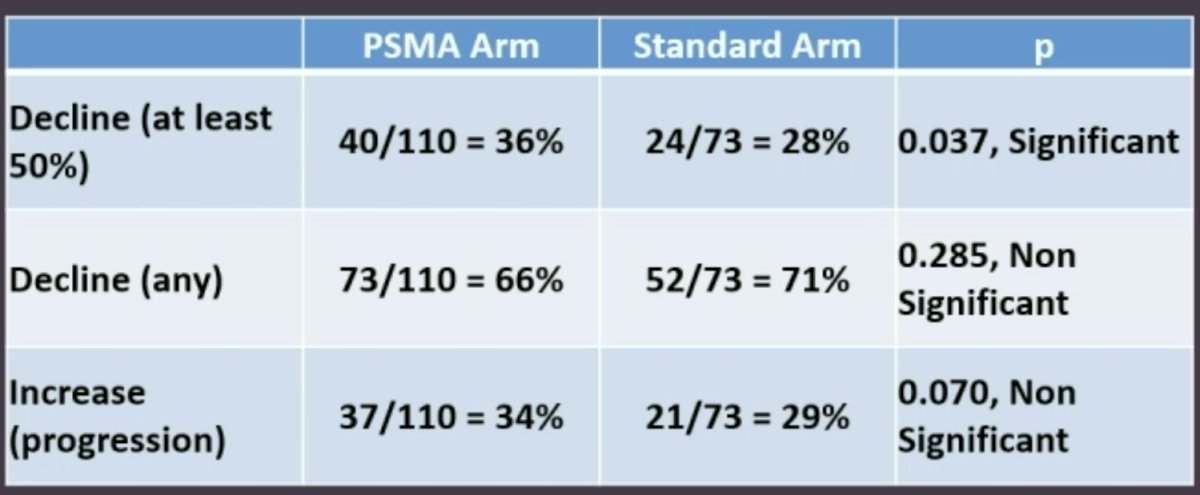
Subgroup analysis in the PSMA arm demonstrated that patients receiving tandem therapy, as opposed to single-agent radioligand therapy (225Ac or 177Lu), had superior overall survival outcomes. The mean survival of patients in this cohort was 11.2 months, while the mean ‘lifespan with satisfied quality’ was 9.5 months.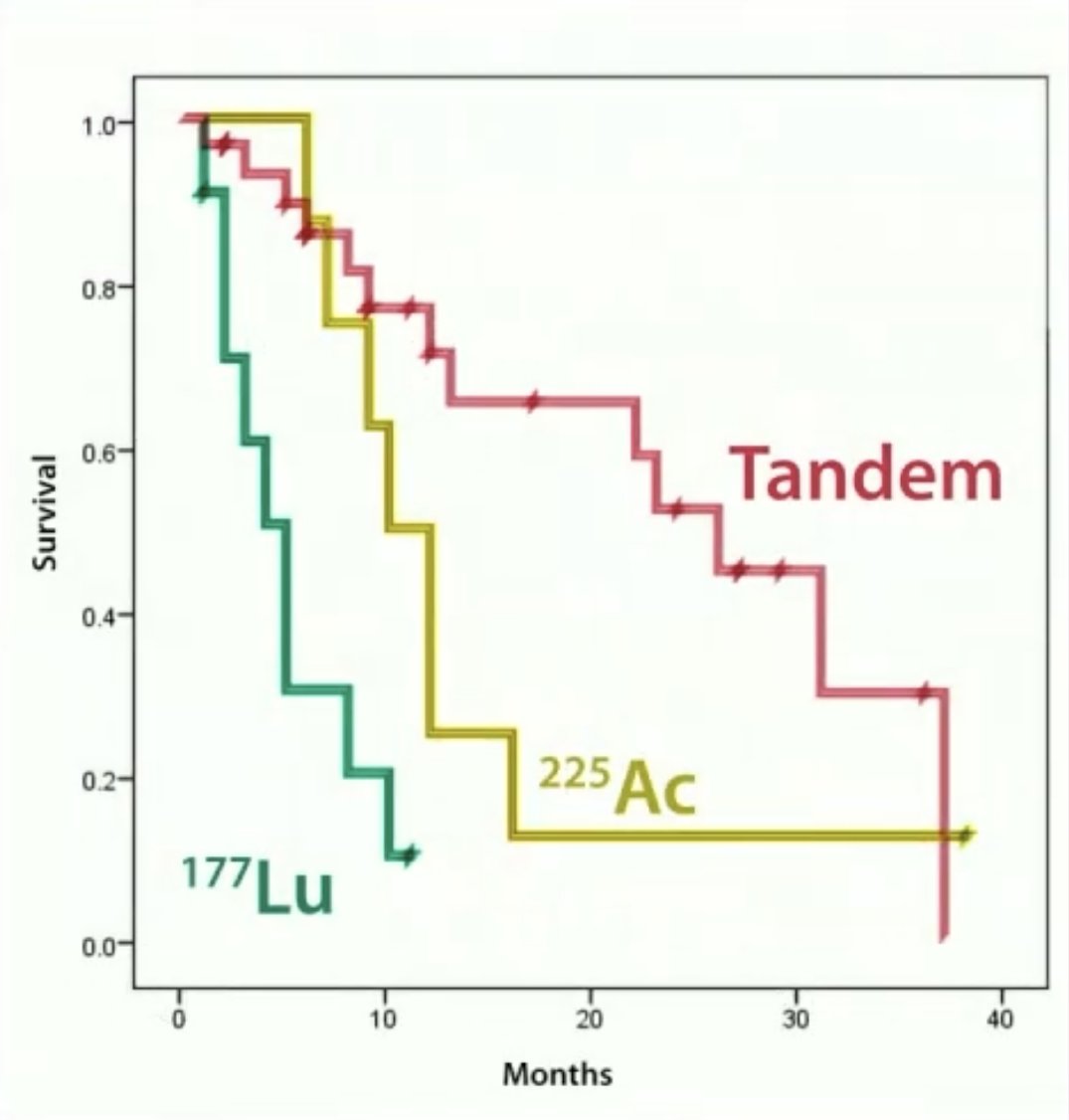
Dr. Novruzov concluded as follows:
- The benefit of PSMA radiolabeled tandem therapy in mCRPC patients has been established for the 1st time in a randomized trial setting.
- Combining alpha (225Ac) and beta (177Lu) radionuclides potentially minimizes adverse effects, while preserving treatment efficacy.
- PSMA radiolabeled therapy opens doors to a ‘brighter future’ for patients, promising to significantly extend the boundaries of disease progression delay.
Presented by: Fuad Novruzov, MD, PhD, Azerbaijan National Centre Of Oncology, Department of Nuclear Medicine, Baky, Azerbaijan
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024
Reference:

