(UroToday.com) The Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024 was host to a prostate cancer therapy session. Dr. Benjapa Khiewvan presented the first Thai experience with Lu-177-PSMA-I&T treatment for patients with metastatic castrate-resistant prostate cancer (mCRPC).
177Lu-PSMA-617 (PLUVICTO®) is currently approved for the treatment of adult mCRPC patients who experience disease progression following treatment with an androgen receptor pathway inhibitor (ARPI) and taxane-based chemotherapy.1 The objective of this study was to evaluate the outcomes of Lu-177 PSMA therapy in a single tertiary institute and determine predictors for treatment outcomes and survival.
Dr. Khiewvan and colleagues retrospectively reviewed data from 40 mCRPC patients referred for treatment with Lu-177 PSMA I&T between August 2019 and December 2023 following disease progression with prior chemotherapy and ADT. Fluorine-18 PSMA-1007 PET/CT was performed in all patients before Lu-177 PSMA I&T treatment and after each treatment cycle. PSA was measured before and after each cycle of Lu-177 PSMA I&T treatment. The study investigators assessed potential prognostic factors such as PSA, standardized uptake value (SUV), metabolic tumor volume (MTV) and total lesion glycolysis (TLG) as predictors of PSA response (PSA decline > 50%) and compared survival in PSA responders (≥50% decline) versus non-responders (PSA decline <50% or rising). Statistical comparisons were performed using the Mann-Whitney U test and time-to-event analyses were performed using Kaplan Meier curve analyses.
Forty patients received 104 cycles of Lu-177 PSMA I&T treatment. The baseline patient characteristics are summarized in the table below.
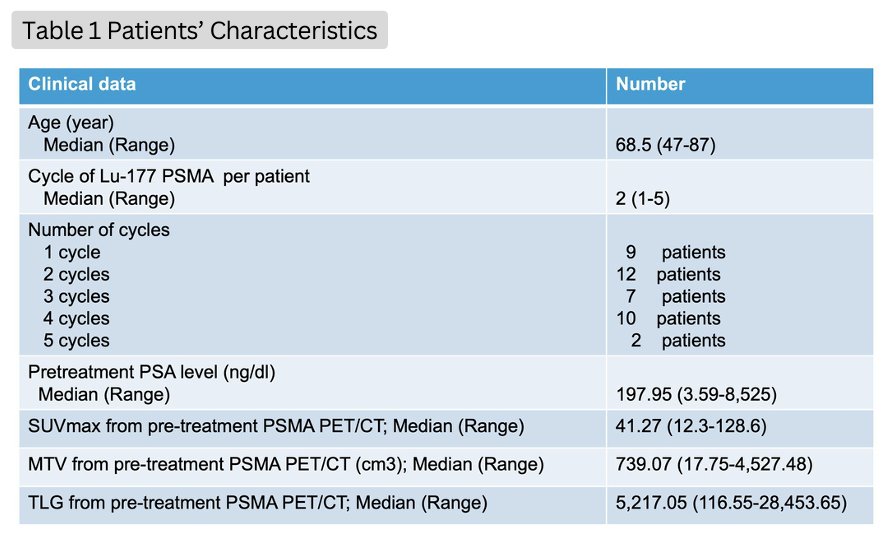
After the 1st cycle of treatment, 25% of patients had a PSA response (i.e., PSA decline ≥50%), with an additional 30% having a PSA decline <50%. After all cycles, the corresponding proportions were 22.5% and 15%, respectively.

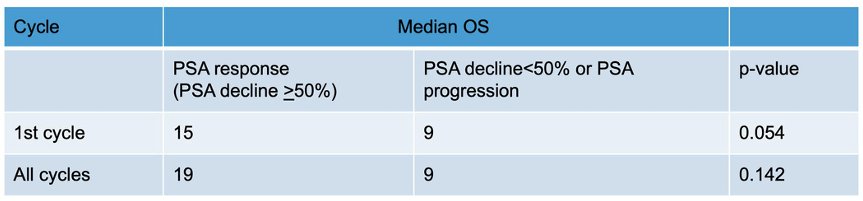
The median overall survival for patients with a PSA response after the 1st cycle or all cycles was significantly longer than those who did not achieve a PSA response.
Pre-treatment MTV significantly predicted PSA response after all cycles (p-value = 0.046). Neither pre-treatment PSA level nor SUVmax predicted PSA response after either the 1ˢᵗ cycle (p-values= 0.36 and 0.72, respectively) or after all cycles (p-values= 0.21 and 0.98, respectively). Pre-treatment TLG did not predict PSA response after 1ˢᵗ cycle (p-value = 0.079) and after all cycles (p-value = 0.081). The median survival time was 10 months (range: 1 to 43 months).
Dr. Khiewyan concluded as follows:
- The initial experience from their institute demonstrated the benefit of Lu-177 PSMA I&T treatment in mCRPC after the failure of ADT and chemotherapy.
- Pretreatment PSA, SUVmax and TLG cannot predict PSA response after the 1st or all cycles.
- Pretreatment MTV can predict PSA response after all cycles but cannot predict PSA response after the 1st cycle.
- Patients with a PSA response after the 1st cycle or all cycles seem to have a longer overall survival compared to those with a PSA decline <50% or progression after treatment.
Presented by: Benjapa Khiewvan, MD, Associate Professor, Department of Radiology (Nuclear Medicine), Bumrungrad International Hospital, Bangkok, Thailand
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024
References:
- FDA approves Pluvicto for metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer. Accessed on June 9, 2024.


