(UroToday.com) The Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024 was host to a prostate cancer therapy session. Dr. David Chen presented the results of a prospective analysis of the ProsTIC registry evaluating the oncologic and quality-of-life outcomes of metastatic castration-resistant prostate cancer (mCRPC) patients with a stable or poor initial PSA response to [177Lu]Lu-PSMA-617, and the prognostic value of baseline imaging biomarkers and dosimetry.
Dr. Chen noted that PSMA radioligand therapy is a ‘maturing’ therapy for mCRPC men with an ever-increasing global experience. PSA is currently used as the primary marker of response. We know, however, that not every patients responds well to radioligand therapy, and when should we stop treatment?

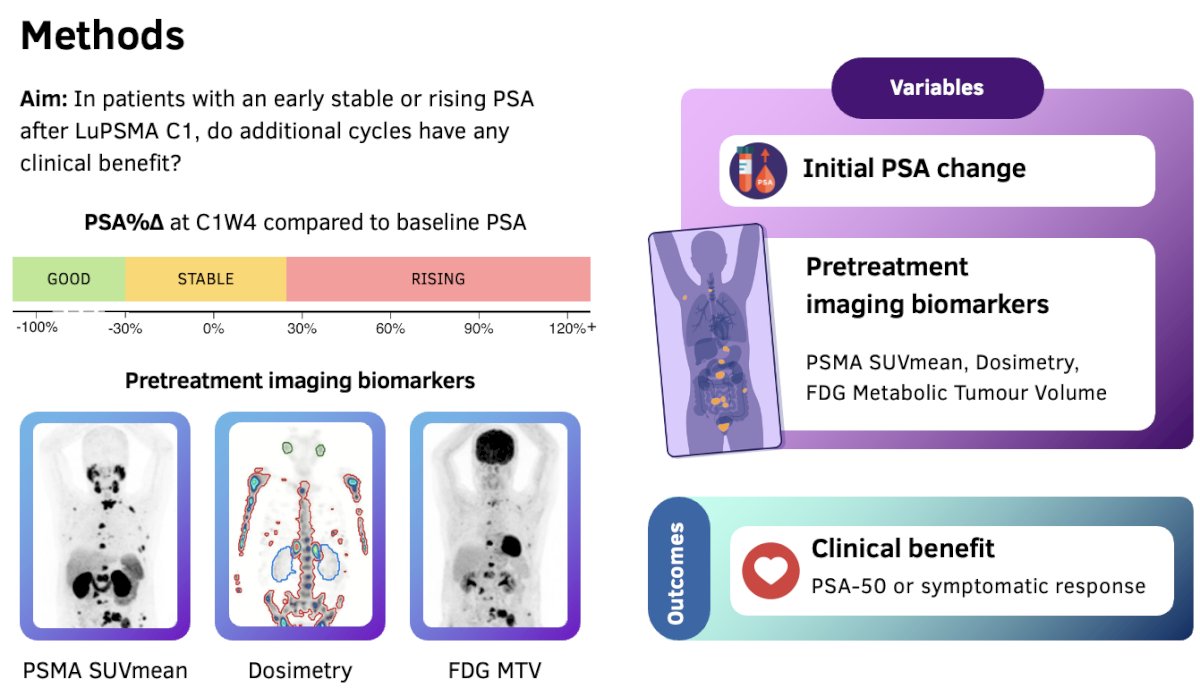
The objective of this study was to evaluate whether additional cycles of LuPSMA have any clinical benefit in patients with an early stable or rising PSA after the 1st cycle of LuPSMA. This analysis included men receiving LuPSMA for mCRPC staring in May 2021. All patients had been pre-treated with (or had been unfit for) taxanes and an androgen receptor pathway inhibitor (ARPI). This represents ‘real-world data’ for safety and efficacy, and all included patients had their care discussed in a multi-disciplinary setting.

The primary variables assessed in this study included:
- % PSA change
- Levels at Cycle 1 Week 4 compared to baseline levels
- Good response: -30% to -100% improvement
- Stable: -30% to + 30%
- Rising: ≥30%
- Levels at Cycle 1 Week 4 compared to baseline levels
- Pre-treatment imaging biomarkers:
- PSMA SUVmean
- Dosimetry
- FDG metabolic tumor volume
- Clinical outcomes
- PSA50 response
- Symptomatic response
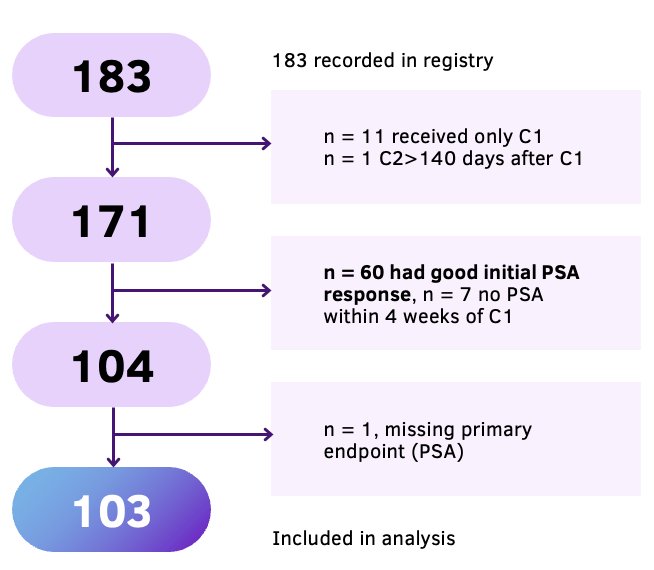
The study investigators identified 103 patients with evidence of a stable or rising PSA after one cycle of LuPSMA who received ≥1 additional cycle of LuPSMA.
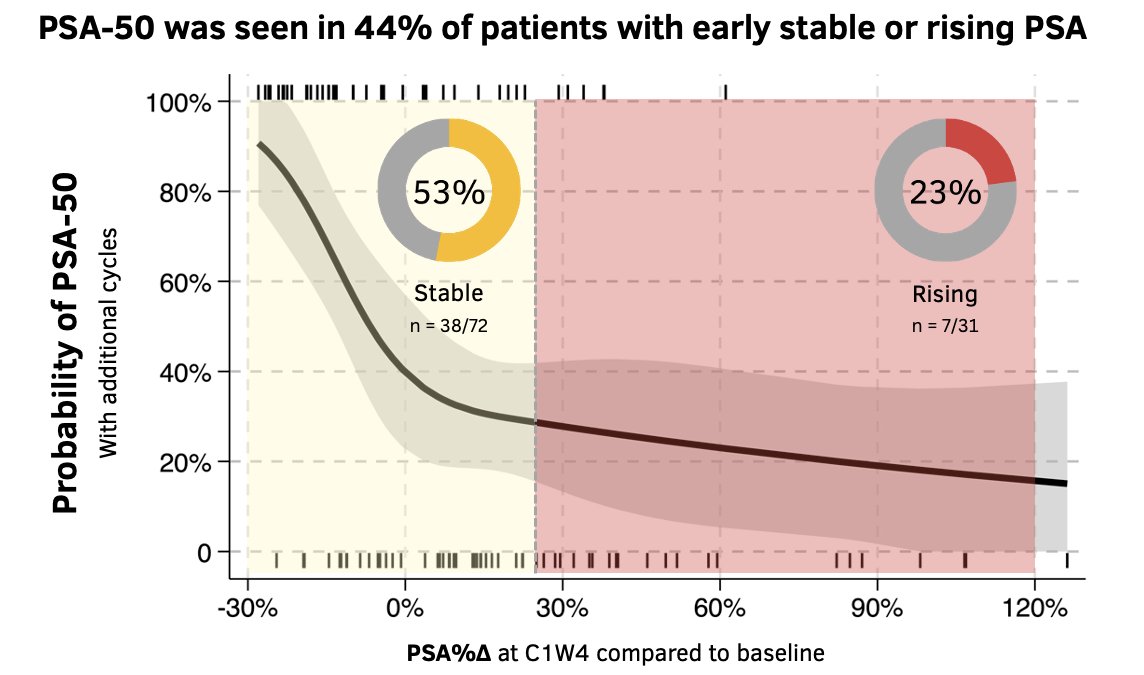
Overall, 44% of such patients with a stable or rising PSA after the 1st LuPSMA cycle achieved a subsequent PSA50 response with additional cycles of LuPSMA. A PSA50 response was observed in 53% of patients with an initial stable PSA response and in 23% of those who had a rising PSA after the 1st cycle.
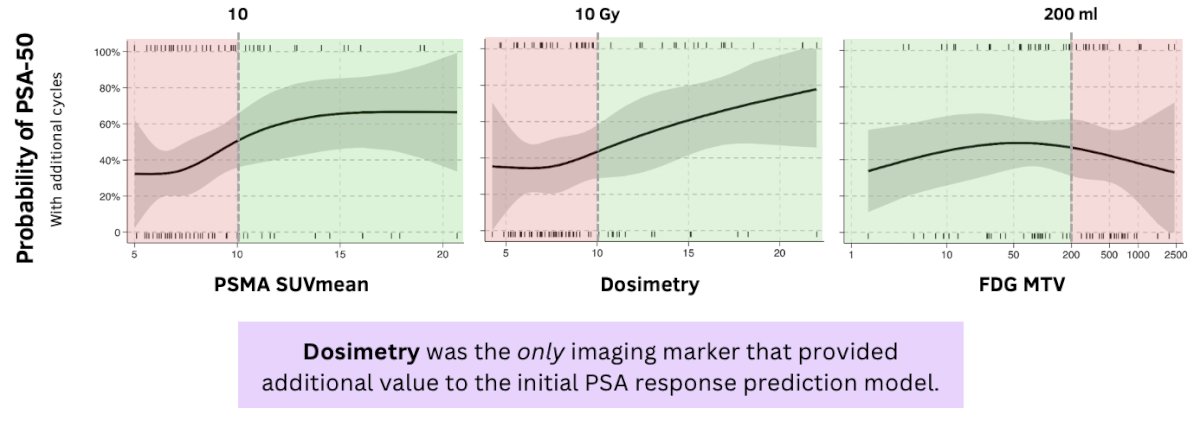
A higher PSMA SUVmean and dosimetry were associated with increased odds of achieving a PSA50 response.
The investigators noted significant quality of life improvements in 71% of the patient cohort, with equivalent proportions irrespective of whether they have an initial stable or rising PSA after the 1st cycle of LuPSMA.
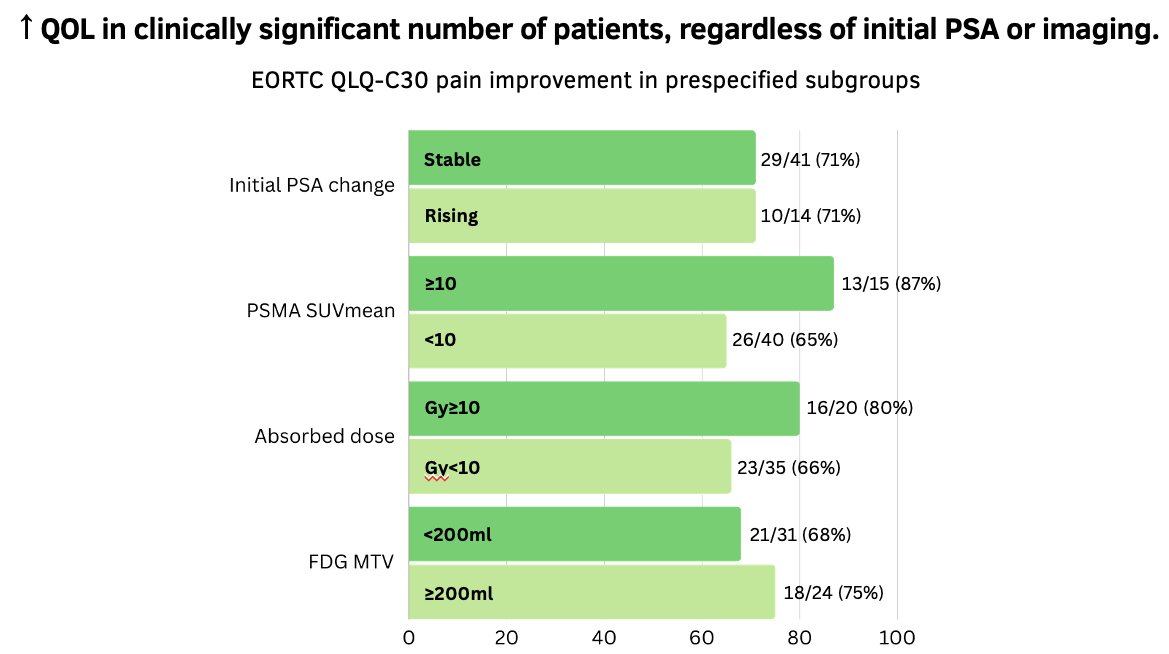
Overall, Dr. Chen concluded that clinicians should continue to treat eligible patients with LuPSMA, irrespective of an early stable/rising PSA or pre-treatment imaging biomarkers.
- 44% of patients achieve a PSA50 response with additional therapy, irrespective of whether they experienced an early stable or rising PSA after the 1st cycle.
- Clinically significant number of patients feel better with additional treatment, regardless of the initial PSA change or imaging findings.
- SUVmean and dosimetry can help predict PSA50 probability.
Presented by: David Chen, PhD, Prostate Cancer Theranostics and Imaging Centre of Excellence, Molecular Imaging and Therapeutic Nuclear Medicine, Cancer Imaging, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024


