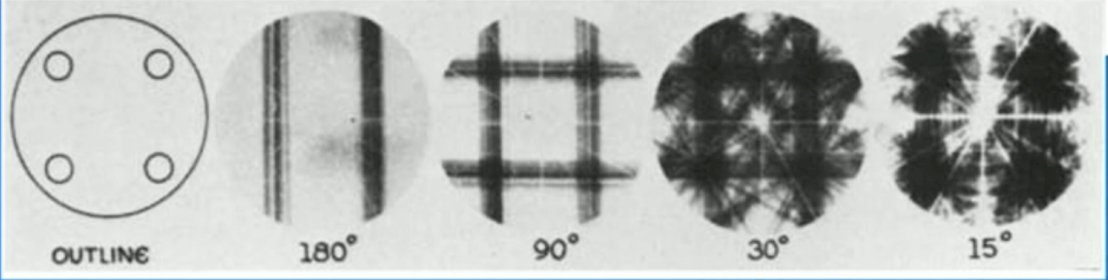
(UroToday.com) The 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) annual meeting featured a session on prostate cancer, and a presentation by Dr. John Kennedy discussing technical considerations for diagnostic, quantitative, and dosimetric imaging for PSMA radioligand therapy in patients with prostate cancer. Dr. Kennedy started by highlighting that radiolabeled small molecules targeting PSMA are used for diagnostic imaging and treatment. This antigen is overexpressed in most prostate cancers but limited in non-prostatic tissues. Nevertheless, clinically, high uptake is observed in the salivary glands, duodenum, and kidneys. Moreover, there is a need for diagnostic, quantitative, dosimetric imaging, such as SPECT, dosimetry, PET, and theranostic pairs/triples. From a historical perspective, the first SPECT acquisition was in 1963, with the small circles of the outline (left) showing the relative location of the four iodine-131 sources, with the other images showing progressive back projection of acquired projections at differing angles:
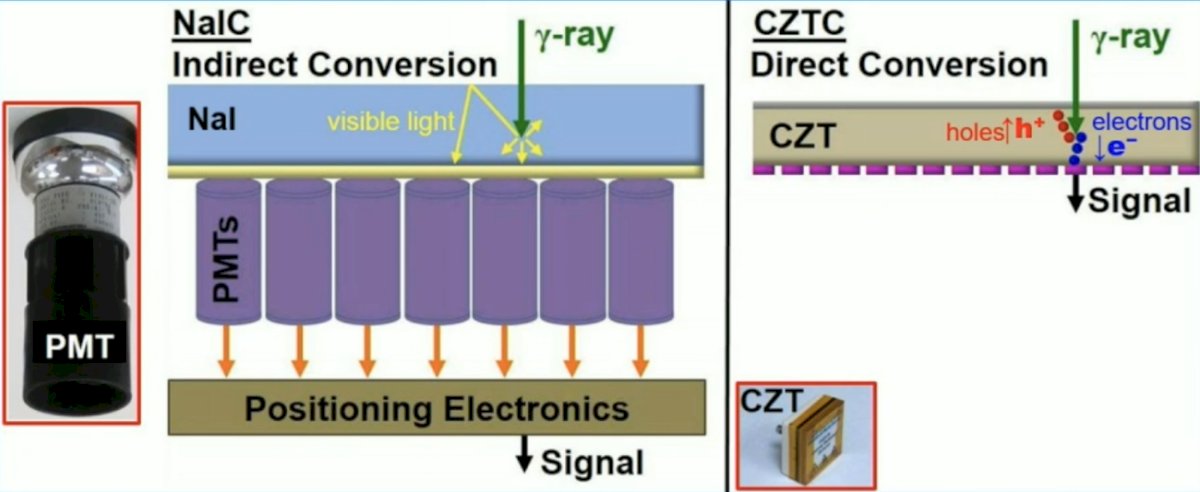
In standard sodium iodide camera detectors, information degrades as it is transferred between mechanisms so both energy and spatial resolution are reduced. In a cadmium zinc telluride (CZT) camera, a semiconductor, enables a direct digitalization of the signal location. Gamma ray production of electron hole pairs allows localized change across a voltage bias:
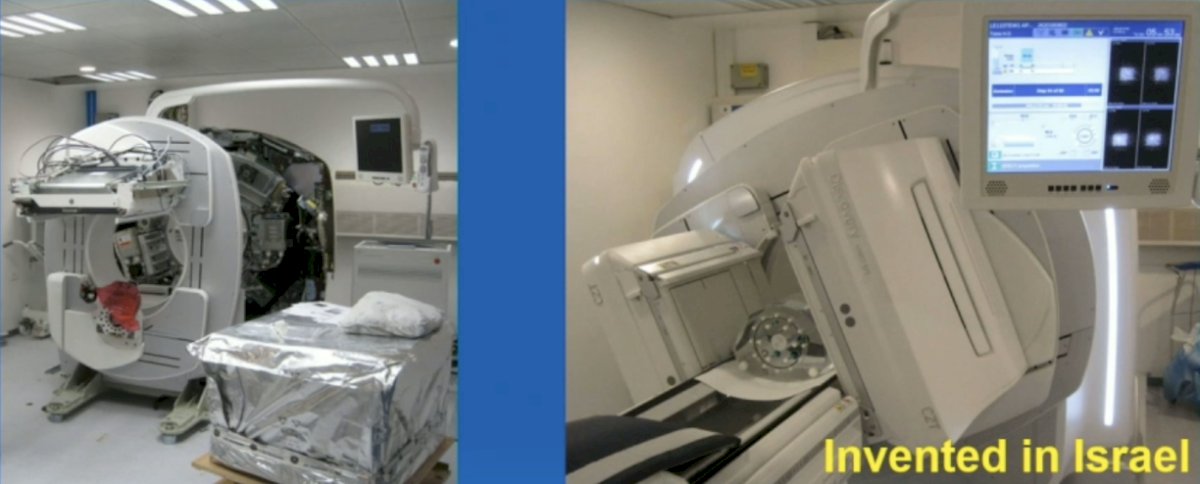
Dr. Kennedy notes that the whole body general purpose solid state SPECT was developed in 2015, which was the first general purpose whole body solid state CZT SPECT/CT (GE Discovery 670 CZT). This was invented in Israel, first installed in September 2015, with the first clinical images being a cardiac MPI (201TI and 99mTc tracers), bone, brain, lung, renal, parathyroid (99mTc tracers), neuroendocrine tumors (MIBG-123I), and metastatic prostate cancer (PSMA-177Lu):
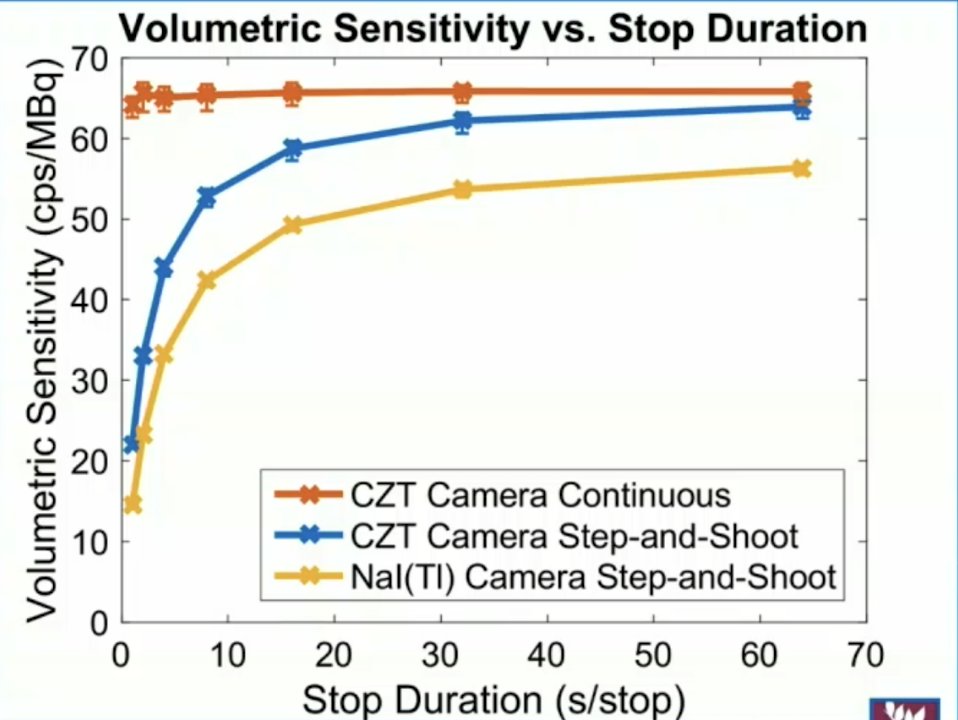
The GE Discovery 670 CZT has 10 x 13 modules (pixel size 2.46 mm), with pixelated detection accommodating better spatial resolution and “near frameless” accommodating brain scans and heart scans. Additionally, better energy resolution accommodates dual isotope scanners and scatter rejection. Segmentation tools facilitate dosimetry, as well as bladder enabled in vitro/in vivo validation. Continuous scan mode means scanning during SPECT rotation, with a volume sensitivity range from 43 to 127 cpm/uCi for a standard camera and from 49 to 142 cpm/uCi for a CZT camera. However, for the continuous scan mode on the CZT camera, it remains within 3% of 146 cpm/uCi, and contract recovery curves are superior with the continuous scan mode:
Several vendors have this option and it may be useful for dynamic scanning. The Veriton whole body solid state SPECT/CT has 12 detector heads that can be rotate to position, with CZT modules mounted on gimbals at the end of cantilevers that can be moved radially (red arrows) to confirm to the patient’s shape. Additionally, swivel motion of the detectors (black arrows) inside the housing provides the required angular projections. This flexibility in detector geometry enables organ specific scanning:
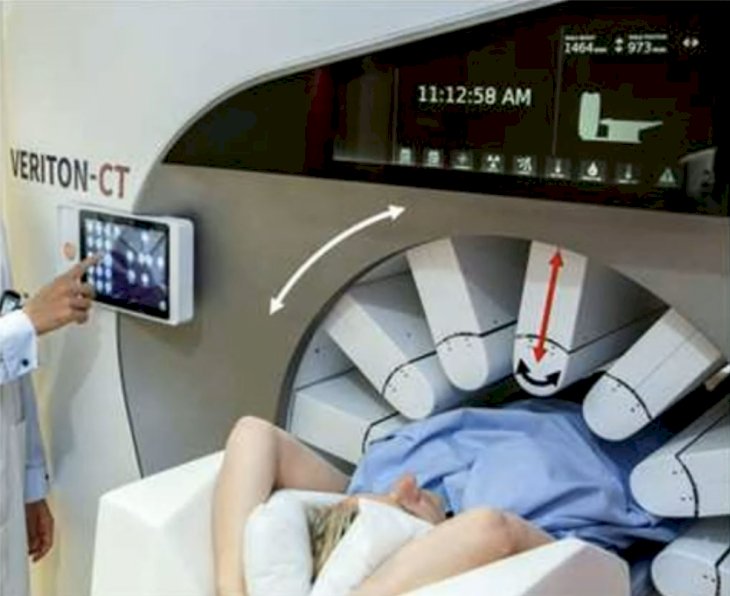
Quantitation with 360 degree CZT Veriton SPECT/CT uses 6 min/bed position, while the Siemens Symbia SPECT/CT uses 16 min/bed position. Nevertheless, SPECT-based dose uncertainties remain high, especially for small lesions:
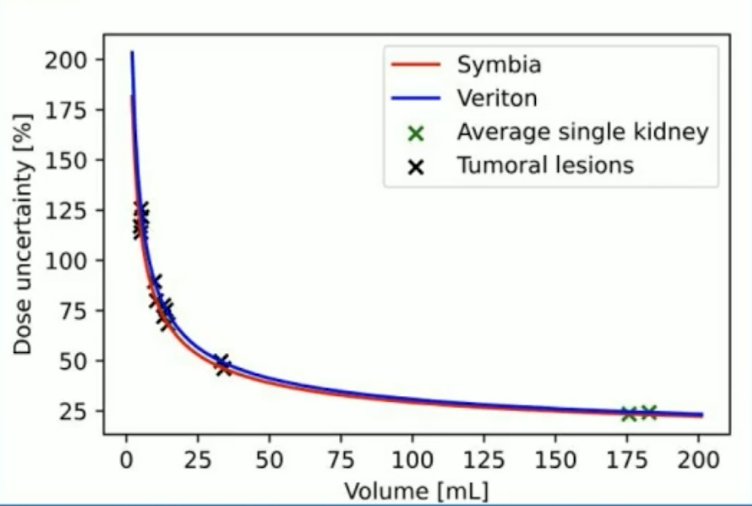
With quantitation with 360 degree CZT SPECT, there are several advantages to same day imaging after 177Lu-PSMA treatment:
- Increased patient comfort and compliance
- Immediate check of extravasation
- Uses time required for patient to be <20 uSv/h @ 1 m for release
Several disadvantages are as follows:
- Low contrast
- A long day for the patient
- Exposes the technician to a higher field
- Increased camera dead time and possible detector saturation affects quantitation in standard Anger gamma cameras, which is less of an issue with CZT
More recently, the AnyScan TRIO SPECT/CT/PET has triple modality imaging, multi-pinhole SPECT technology, Monte Carlo reconstruction, modelled scattered correction, LYSO, and time of flight:
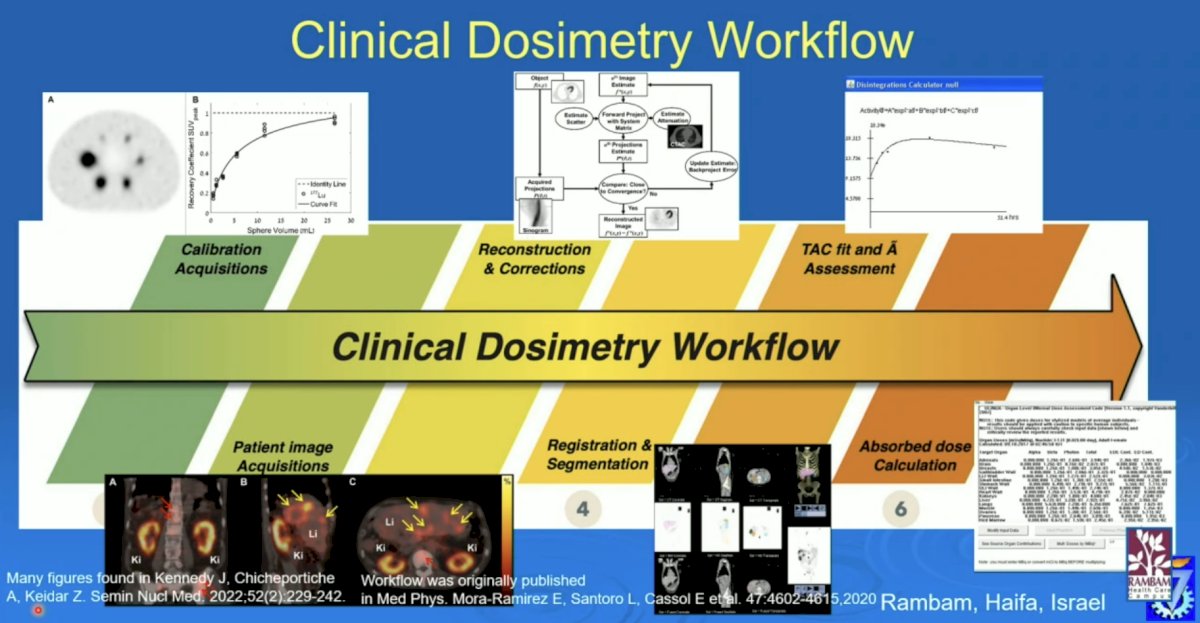
The clinical dosimetry workflow includes calibration acquisitions, patient image acquisitions, reconstruction and corrections, registration and segmentation, TAC fit and assessment, and absorbed dose calculation:
Single time point dosimetry includes assessing cumulative activity in individual organs and tissues that can be determined by a time integration of activity as determined by several imaging sessions (planar or SPECT). The following illustration is of a single-time point measurement at 90 hours, for an organ and population where the effective half time is expected to be hear 50 hours (red curve):
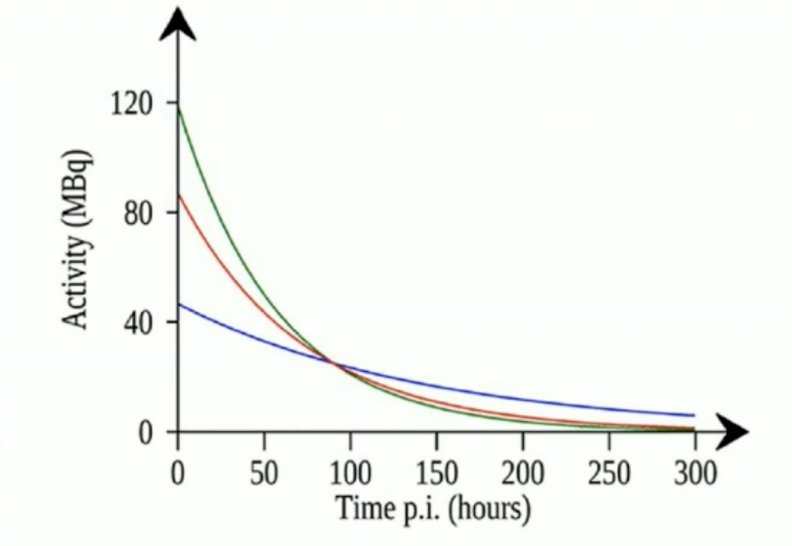
There are several problems with multiple time point dosimetry, including:
- Patient time and discomfort
- Camera time
- Technician imaging time
- Segmentation and analysis
- Registration
So, can a single time point give the same or close enough information? Of note, the individual time information is lost and has to be assumed for various organs and tissues in a population. Importantly, at the optimal imaging time point, large errors in the estimate of the time information (decay rate constants) cause only modest errors in the absorbed dose calculations:
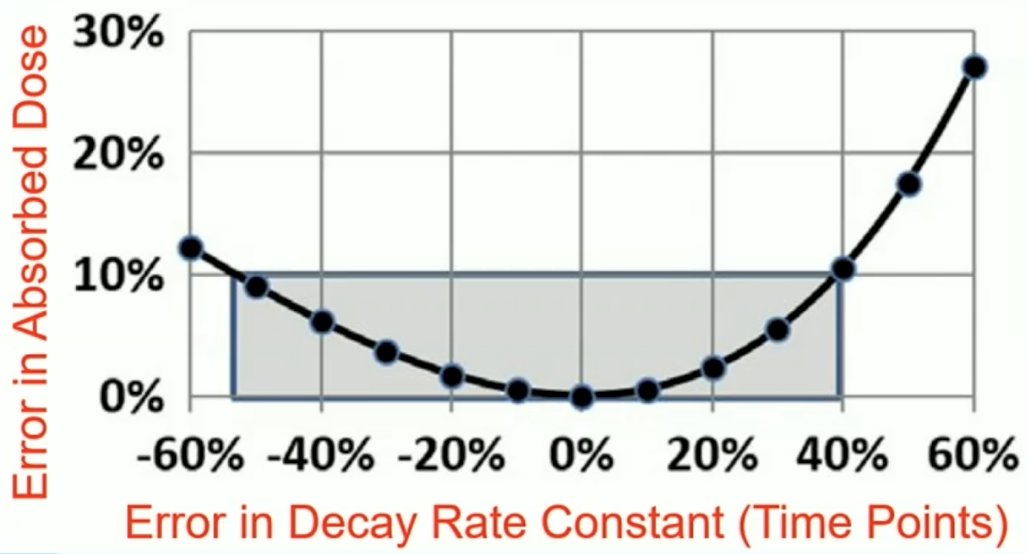
Dr. Kennedy notes there are several problems with single time point dosimetry:
- The magnitude depends on a single time point, if it is not averaged over several imaging sessions, which reduces the error
- The effective decay rate constant is estimated from previous populations, not individually determined, so:
- An error is introduced
- Also, the absorbed dose rate is unknown
- Consequently, metrics which account for the dependence of radiobiological responses on absorbed dose rate cannot be determined
- For PSMA, the real value of dosimetry might be in the dose response of the tumor, and it remains to be seen if time-activity curves for tumors can be generalized
Dr. Kennedy then discussed dosimetry and lesion background ratio, noting that SPECT quantitation has other challenges. Ideally, the camera’s calibration factor is constant: counts per activity. However, scatter and other effects lead to geometry dependent results, including the calibration factor depending on the lesion to background ratio. Low contrast features will have doses underestimated if the camera was calibrated with high contrast features, and this inaccuracy is more pronounced with smaller features:

Scatter is primarily from Compton scatter within the object or with the detector, blurring features since ray paths are not straight. The aim of scatter correction is to remove the scattered photon contribution from the energy peaks used for imaging:
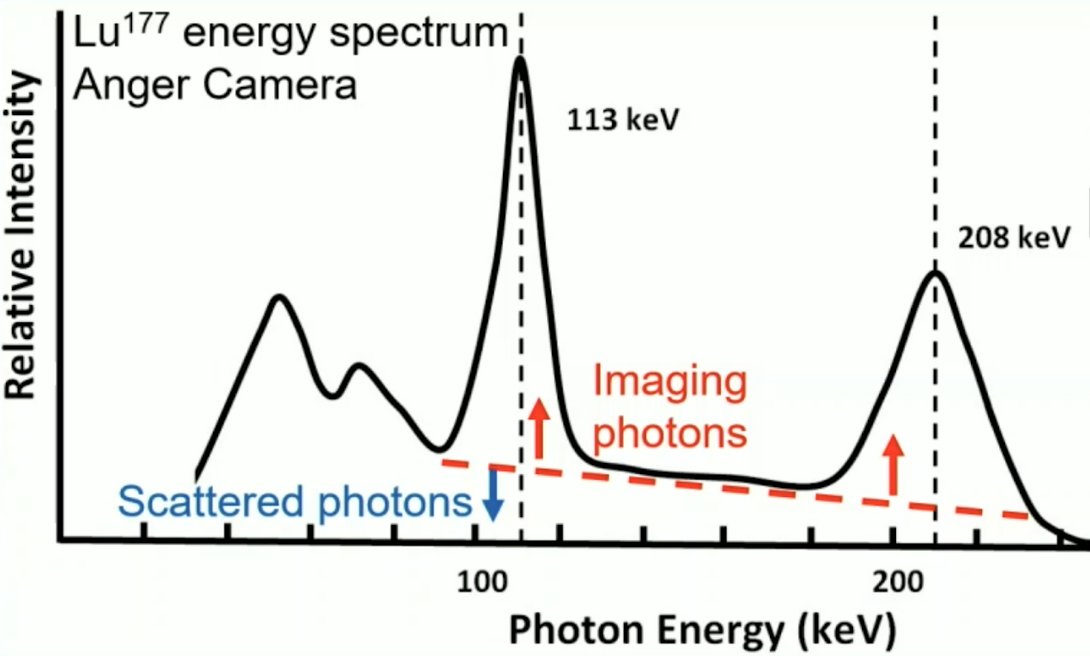
TEW is a weighted average of the scatter estimate counts (blue), if removed from the counts within the peak (red). Scatter is estimated from adjacent energy windows on either side of the peak:
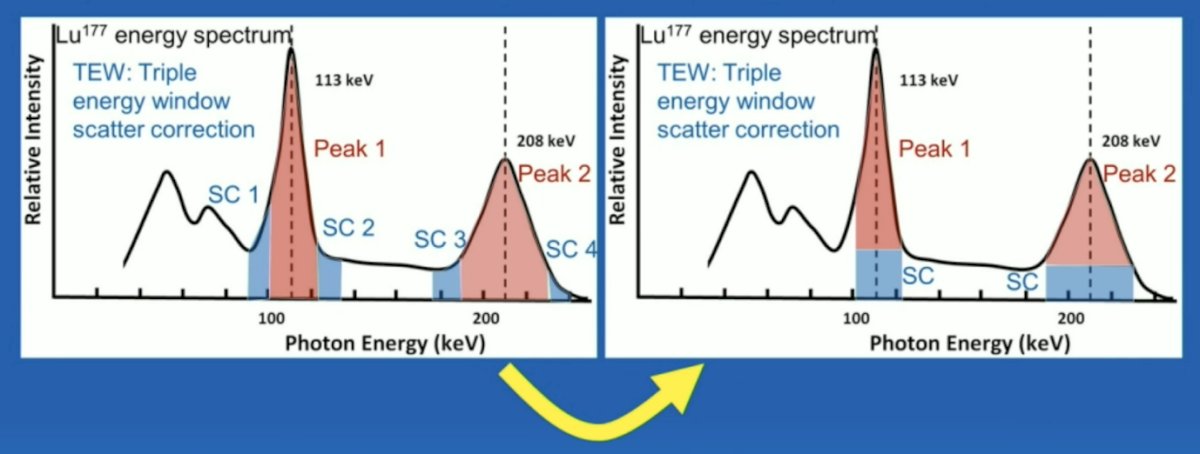
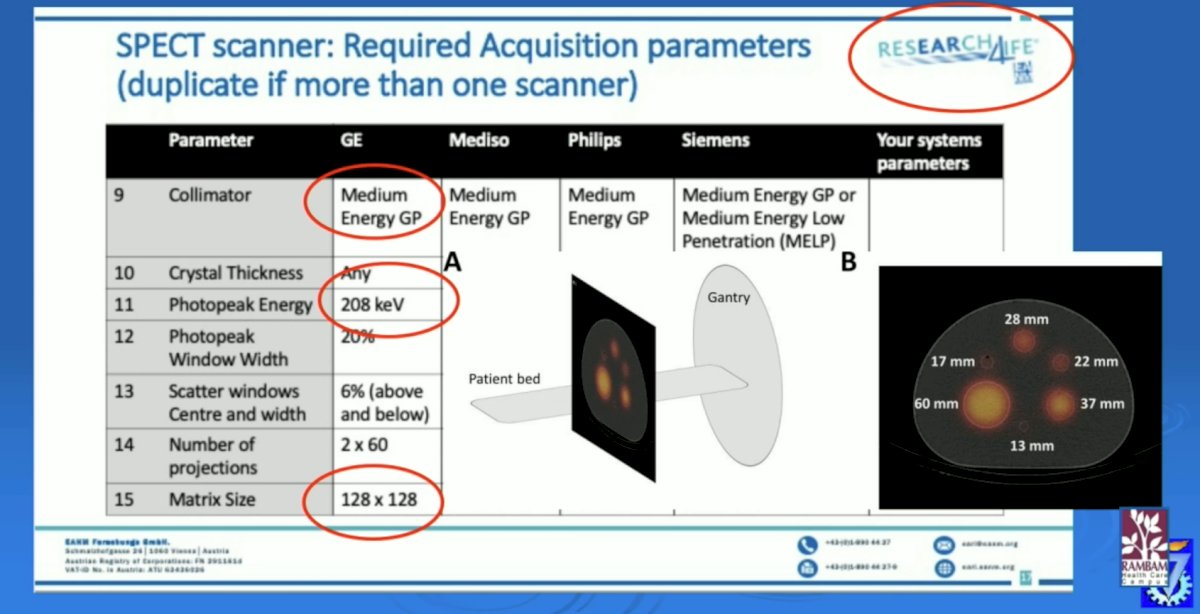
The SNMMI has developed a harmonization protocol for SPECT/CT scanner calibrations, allowing camera calibration for quantitation, with NIST traceable sources, and 10 sites already accredited for 177Lu.
Violet and colleagues [1] previously assessed 177Lu-PSMA-617’s radiation dosimetry and relationships to pre-therapeutic imaging and outcomes among 30 patients within a prospective clinical trial. This study found that the mean absorbed dose to kidneys, submandibular and parotid glands, liver, spleen, and bone marrow was 0.39, 0.44, 0.58, 0.1, 0.06, and 0.11 Gy/MBq, respectively. Median whole-body tumor-absorbed dose was 11.55 Gy and correlated with PSA response at 12 weeks. A median dose of 14.1 Gy was observed in patients achieving a PSA decline of at least 50%, versus 9.6 Gy for those achieving a PSA decline of less than 50% (p < 0.01). On screening PSMA PET, whole-body tumor SUVmean correlated with mean absorbed dose (r = 0.62), and SUVmax of the parotids correlated with absorbed dose (r = 0.67). However, there was an inverse correlation between tumor volume and mean dose to the parotids (r = -0.41) and kidneys (r = -0.43):

Dr. Kennedy notes that uptake of radionuclinde in normal tissues is lower in patients with a high tumor burden because of a tumor sink effect. There is a reduction of ~40% dose to the kidneys for burdens > 1,355 mL 68Ga-PSMA uptake compared to negative scans. Thus, patients may benefit from increased therapeutic activity without exceeding the radiation dose limit for organs at risk.
There are several solid-state PET scanners, including:
- The Philips Vereos: which was manufactured in Israel
- Siemens Vision: which has the best commercial temporal resolution of 204 ps, which translates to a time of flight localization of ct/2 = 3 cm
- GE MI: which has the highest count rate sensitivity
State of the art PET/CT at Rambam includes the first solid state digital BGO PET/CT, the first clinical installation in November 2021, the first clinical images obtained, and the first Rb-82 dBGO cardiac scans:
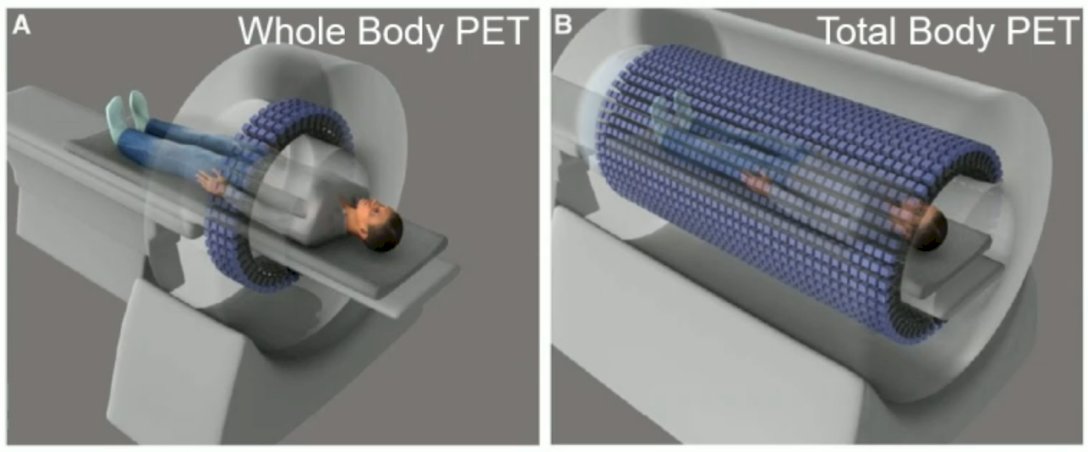
A total body PET encompasses the entire body allowing imaging of all the tissues and organs of the body simultaneously. The count rate sensitivity increases by a factor of about 40 for imaging the entire body, with 1/40 the scanning time or 1/40 the injected activity. The world’s first total-body PET scanner is the EXPLORER:
PET diagnostic quantitation sums the total lesion volume within the bone, extracting the SUVmean and SUVmax. This is an automatic and reproducible process, segmenting bone on CT and masking PET. With regards to PET/MR, PSMA + MRI has superior sensitivity in detecting clinically significant prostate cancer compared with mpMRI. For PET/SPECT radiopharmaceuticals, theranostics can be monitored by performing PET or SPECT. For 225Ac, imaging can be done directly, with 440-keV (213Bi) and 218 keV (221Fr) photo peaks. Theranostic pairs can be developed such as the use of gamma ray emitting radiolanthanides with a corresponding therapeutic form. Terbium-155 (155Tb), a gamma ray, can be used for imaging, along with a therapeutic terbium isotope, such as 161Tb, presenting an ideal “theranostic pair” because of virtually identical chemical properties.
Dr. Cohen concluded his presentation discussing technical considerations for diagnostic, quantitative, and dosimetric imaging for PSMA radioligand therapy by highlighting that many technological developments in nuclear medicine enable shorter scan times, larger fields-of-view, and more accurate image quantification and localization, facilitating diagnosis and treatment with PSMA radiopharmaceuticals.
Presented by: John A. Kennedy, PhD, Rambam Health Care Campus, Haifa, Israel.
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024
References:
- Violet J, Jackson P, Ferdinandus J, et al. Dosimetry of 177Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: Correlations Between Pretherapeutic Imaging and Whole-Body Tumor Dosimetry with Treatment Outcomes. J Nucl Med. 2019 Apr;60(4):517-523.


