(UroToday.com) The 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) annual meeting featured the Therapy Center of Excellence Saul Hertz Lecture and Award, and a presentation by Dr. Michael Hofman discussing bridging evidence-based medicine and precision oncology. Saul Hertz (1905-1950) conceived and brought from bench to bedside radioactive iodine for medical uses based on the 1936 question “Could iodine be made radioactive artificially?” This was followed by the 1937 radioactive iodine studies assessing thyroid physiology, tracer qualities, dosimetry, and thyroid carcinoma. On March 31, 1941, there was the first report of therapeutic use of radioactive iodine based on a series of 29 patients.
The Saul Hertz Award was established in 2016 soon after the launch of the SNMMI Therapy Center of Excellence, based on a Lifetime Achievement Award in honor of the professional achievements of Dr. Hertz as the pioneer in Radioiodine Therapy. This year’s recipient of the Saul Hertz Award is Dr. Michael S. Hofman, who has received ~$35 million in research funding as a Chief Investigator since 2012. To date, his research has 18,500+ citations, (84% in the last 5 years), > 280 publications, 45 publications with >100 citations, h-index of 64, and on ExpertScape he is #1 for Lutetium and #2 for PET/CT.
Dr. Hofman notes that radio-iodine after >80 years is still the best theranostic to date, noting that for metastatic thyroid cancer, patients can be imaged with 124I PET or 123I/131I SPECT, can be treated with 131I and can achieve >300+ Gy to the tumor. As follows is Hofman’s Hierarchy of Theranostics, noting that compounds with low uptake will not improve patient outcomes, PSMA & DOTATE are game changers (early, combined, and personalized), and there is hope that the next theranostic can still beat iodine and provide curative theranostics: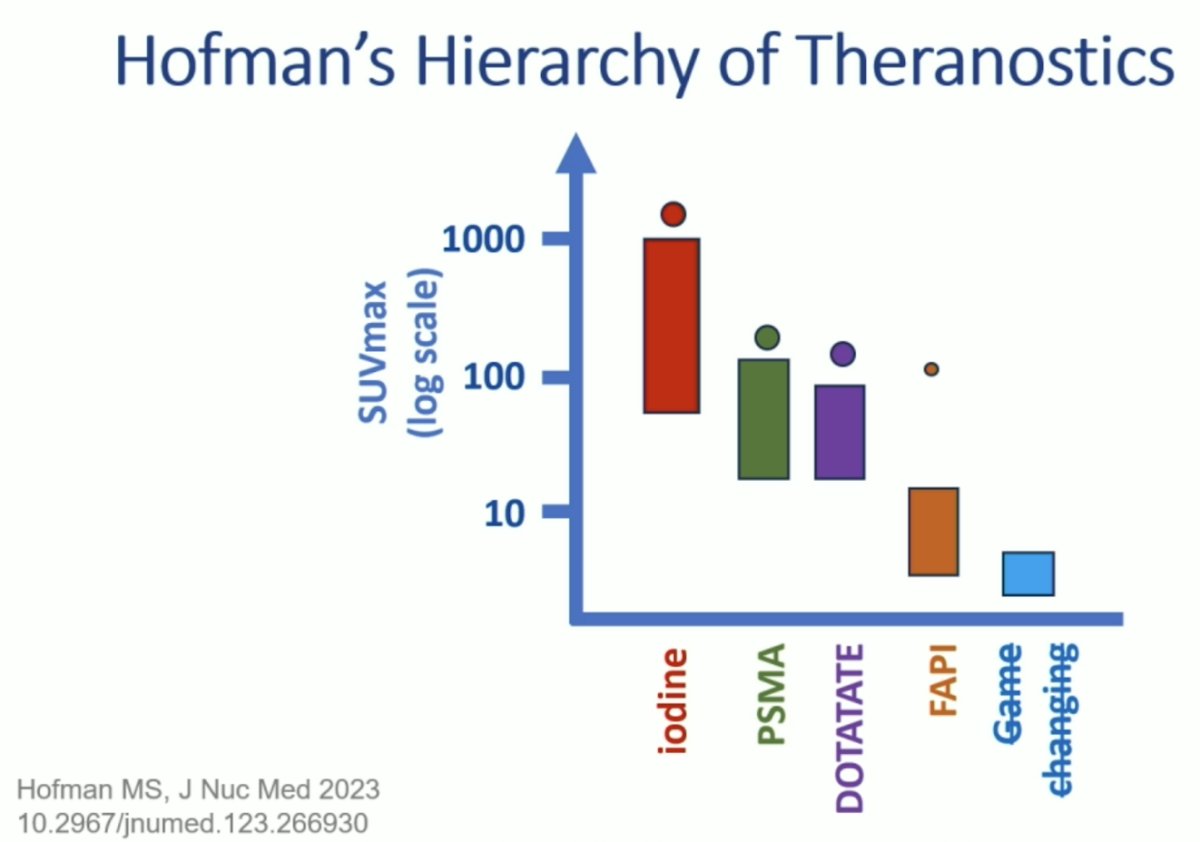
Dr. Hofman’s theranostic journey started in 2005 when he attended the EANM annual congress and noted presentations on 177Lu-DOTA0, Tyr3 therapy, and 68Ga-DOTA-Tyr3-octreotide-PET as a tracer of choice for staging neuroendocrine tumors. His team then ushered in 68Ga-DOTATE PET for imaging neuroendocrine tumors in 2012, which was eventually funded in Australia in 2018. Since then, there has been an explosion of availability of 68Ga-DOTATE PET in Australia, starting with one center in 2010 and now up to 116 centers in 2024:
Dr. Hofman notes that in 2012 he and his team thought that PET could replace most/all of conventional nuclear medicine, with superior image quality and faster acquisition. Moreover, he thought it was cost-effective, with flexible acquisition protocols, high-resolution nuclear medicine, and new tracers providing new indications. 177Lu-DOTATE in neuroendocrine tumors subsequently followed the PET imaging, with exceptional responses noted in metastatic gastrinoma, metastatic paraganglioma, pediatric refractory neuroblastoma, and metastatic cervical small cell carcinoma in combination with etoposide. It was at this point in 2013 that Dr. Hofman realized that while personalized medicine may be optimal, “high level” evidence and registration was needed and prospective data critical in order to trigger acceptance by oncologists. Thus, he was convinced that randomized controlled trials were the only way to convince oncologists and funders, not case reports/case series. This is evident in the Peter Mac theranostic program of trials and publications, noting the prospective trials/data from 2016 onward, particularly for 177Lu-PSMA and 177Lu-DOTATE: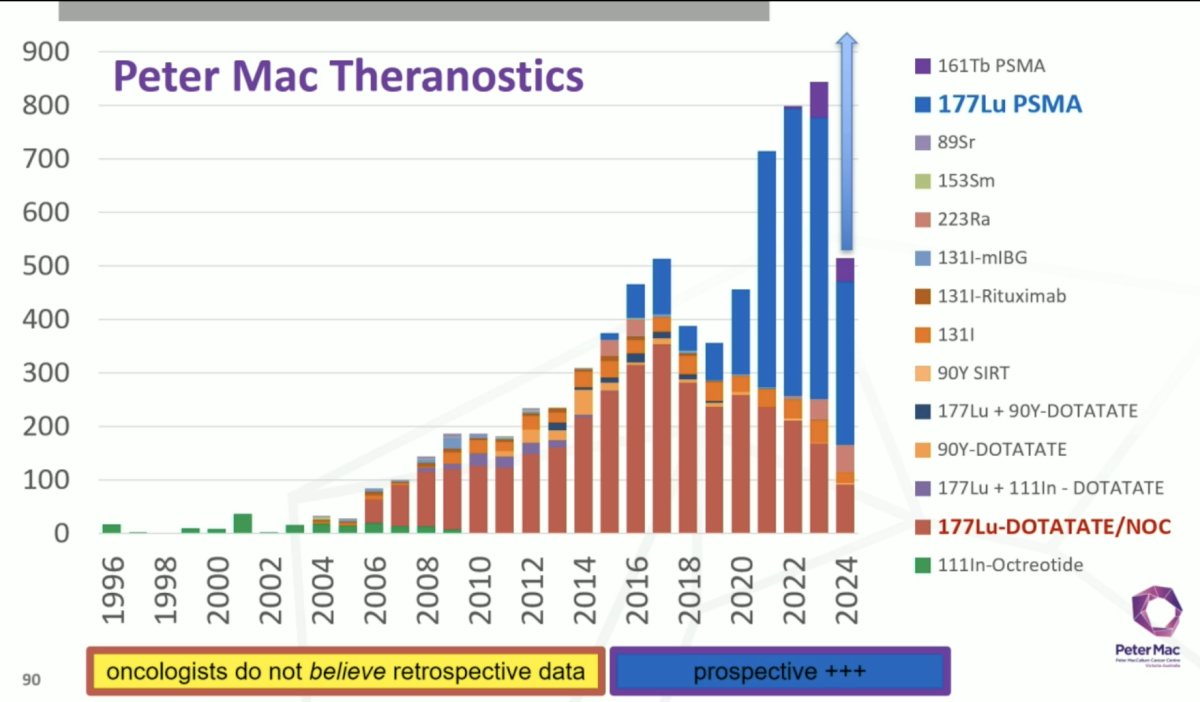
The first PSMA PET/CT scan was done at Peter Mac in 2014 on a patient with Gleason 9 prostate cancer with normal conventional imaging. His PSMA PET/CT showed bone metastasis, a 3 mm lymph node, and primary prostate cancer:
Again, Dr. Hofman felt that a registration trial was necessary, thus the concept of the proPSMA trial was born in 2015, followed by a detailed proposal, trial launch, recruitment and analysis, and publication in 2020.1 proPSMA was a multi-center randomized controlled trial of men with histologically confirmed prostate cancer who were being considered for curative intent radical prostatectomy or radiotherapy. All patients had at least one high-risk factor including serum PSA ≥ 20 ng/mL, Grade Group 3 to 5 disease, or clinical stage T3 or greater. Following enrollment, patients were randomly assigned in a 1:1 ratio to either conventional imaging consisting of bone scan and CT or 68Ga-PSMA-11 PET/CT. Between 2017 and 2018, 302 patients were randomized to either conventional imaging (n = 152) or 68Ga-PSMA-11 PET/CT (n = 150). PSMA PET/CT demonstrated a 27% absolute greater AUC for accuracy compared to conventional imaging (92% versus 65%) for the detection of either pelvic nodal or distant metastatic disease. Conventional imaging had both a lower sensitivity (38% versus 85%) and specificity (91% versus 98%). Subgroup analyses by site of metastasis demonstrated the superiority of PSMA PET/CT for pelvic nodal (AUC: 91% versus 59%) and distant metastases (AUC: 95% versus 74%):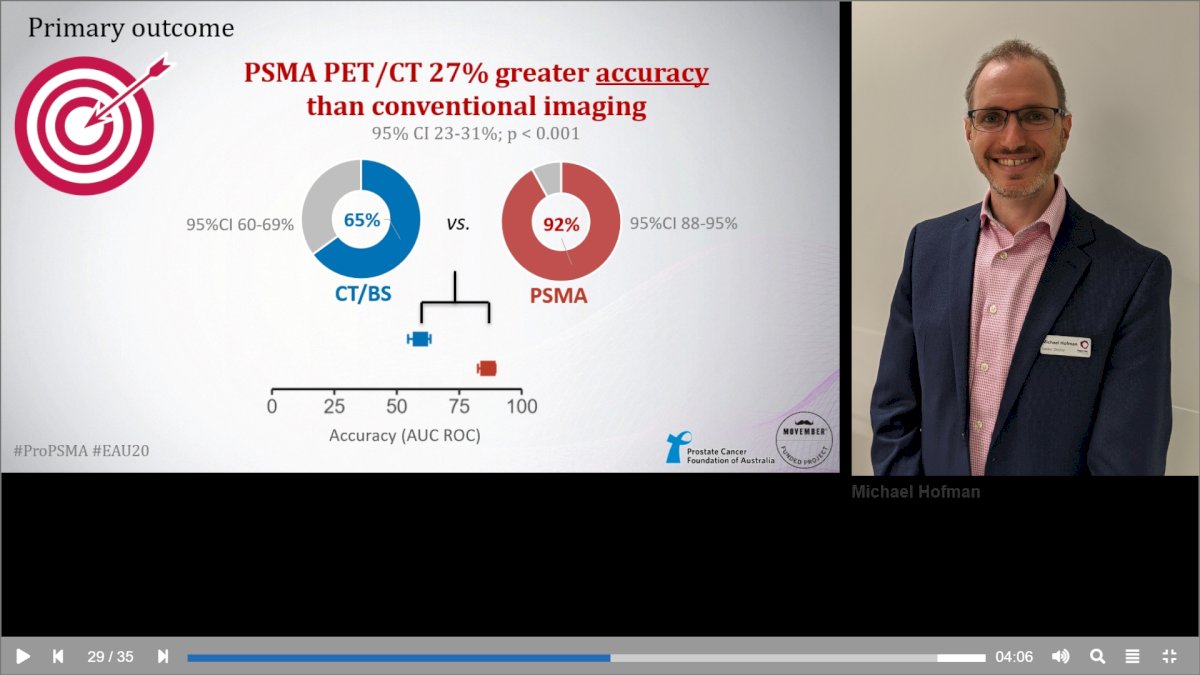
Since then, this publication has more than 1,390 citations (top 10% of Lancet). Following publication of this trial, Dr. Hofman noted “Establishing collaborative networks, working together, and upskilling the next generation of specialists in clinical trial methodology are key to achieving this goal. As new radiotracers emerge, there is a pressing need to develop and enroll patients in well-designed clinical trials. These are our best chance to properly evaluate the impact of our tests and enable widespread acceptance.”
Dr. Hofman notes that the first results of 177Lu-PSMA-I&T were presented at the 3rd World Congress in Theranostics in March 2015, with data on 37 patients showing best PSA response in the following figure: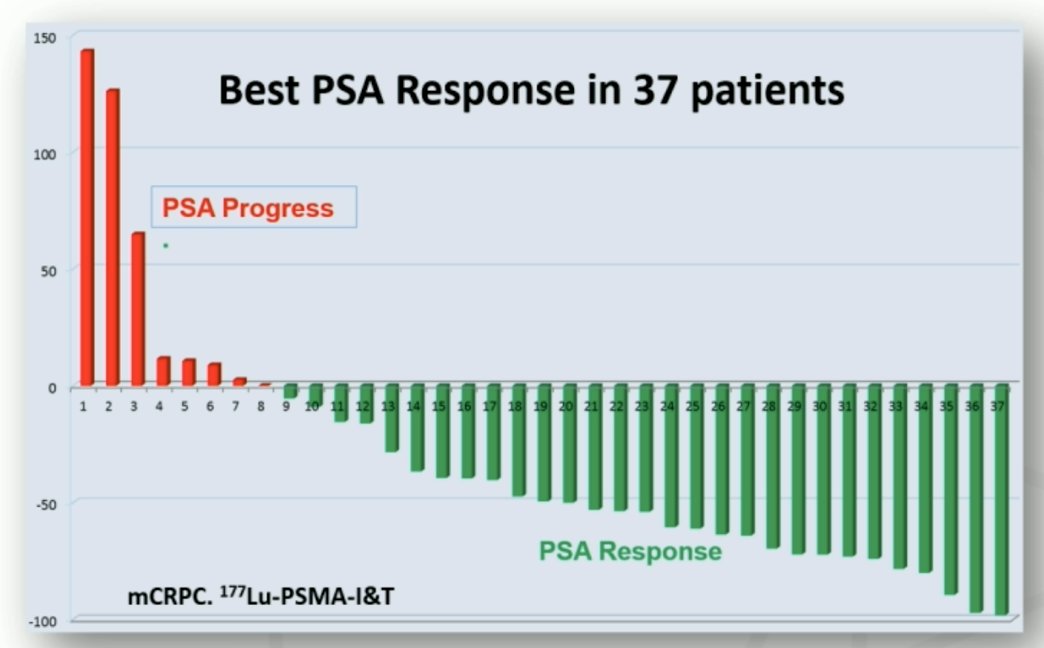
Following this, Dr. Hofman’s group published results of the LuPSMA single-center, single-arm, phase 2 study,2 showing that in 30 patients there was a PSA50 response in 64% of patients leading the way for prospective trials to attempt to change clinical paradigms:![Dr. Hofman’s group published results of the LuPSMA single center, single arm, phase 2 study [2], showing that in 30 patients there was a PSA50 response in 64% of patients leading the way for prospective trials to attempt to change clinical paradigms](/images/com-doc-importer/169-snmmi-2024/snmmi-2024-saul-hertz-s-theranostic-dream-is-shaping-the-future-of-cancer-care-bridging-evidence-based-medicine-and-precision-oncology/image-6.jpg)
This led to the TheraP trial, which was the first randomized clinical trial of LuPSMA.3 TheraP was the first randomized study to evaluate 177Lu-PSMA-617 vs cabazitaxel for men with mCRPC after docetaxel. To screen into the study, all men had both 68Ga-PSMA-11 and 18F-FDG PET/CT and were required to have high PSMA-expression (at least one site with SUVmax ≥ 20) and no sites of FDG-positive/PSMA-negative disease. All patients had progressive disease with rising PSA ≥20 ng/mL after docetaxel and 91% had received prior enzalutamide or abiraterone. Overall, 200 patients were randomized 1:1 to 177Lu-PSMA-617 at a dose of 6-8 GBq every 6 weeks for up to 6 cycles of therapy or cabazitaxel 20 mg/m2 every 3 weeks for up to 10 cycles. Patients were stratified based on disease burden and prior anti-androgen therapy. The primary endpoint of this study was a PSA decline of 50% (PSA50) and secondary endpoints included PSA-PFS and overall survival. After a median follow-up of 13 months, 177Lu-PSMA-617 significantly improved PSA-PFS compared with cabazitaxel (HR 0.63, 95% CI 0.46 to 0.86) and had a much higher PSA50 rate (66% vs 37%): 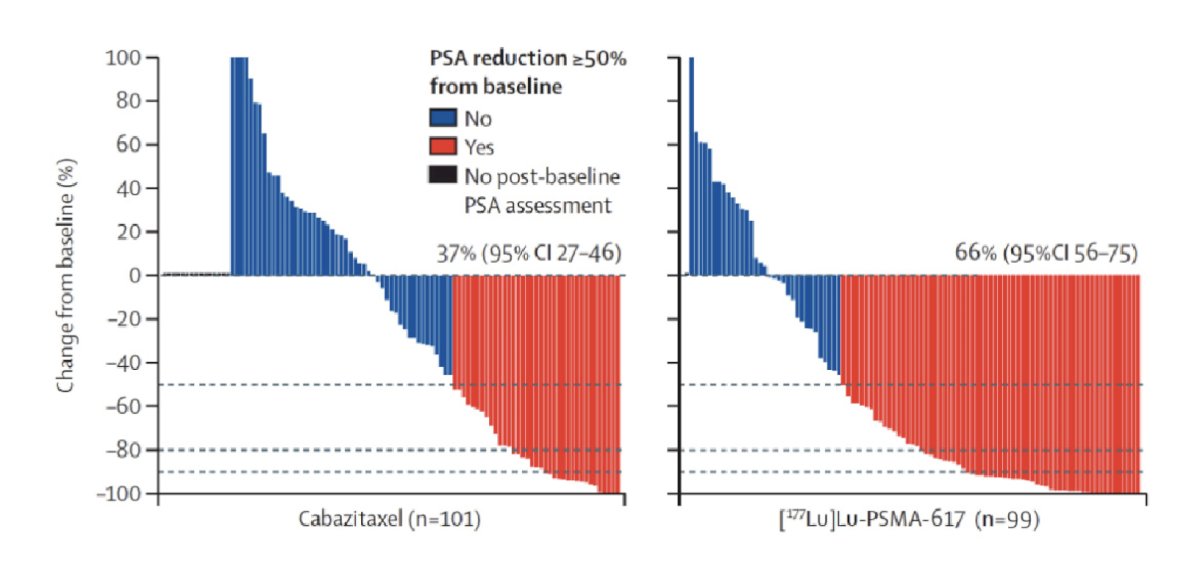
Recently, Hofman and colleagues4 noted that after a median follow-up of 35.7 months (IQR 31.1 to 39.2), 77 (78%) participants had died in the 177Lu-PSMA-617 group and 70 (69%) participants had died in the cabazitaxel group. Overall survival was similar among those assigned to 177Lu-PSMA-617 versus those assigned to cabazitaxel (restricted mean survival time 19.1 months vs 19.6; difference -0.5 months; p = 0.77):
Although there is no survival benefit for 177Lu-PSMA-617 versus cabazitaxel, Dr. Hofman notes that 177Lu-PSMA-617 increases patient-reported outcomes and improves deterioration-free survival compared to chemotherapy: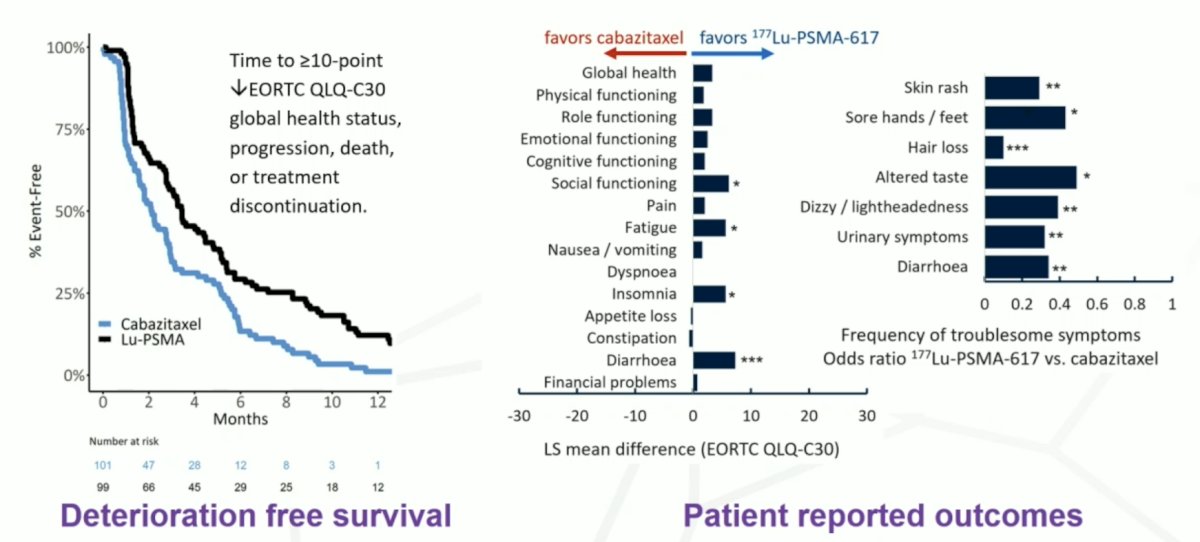
Since TheraP, the Prostate Cancer Foundation has been instrumental in funding and developing the Prostate Cancer Theranostic & Imaging Center of Excellence (ProsTIC) at Peter Mac, with a pillar of clinical trials, discovery research, and education and leadership:
Dr. Hofman notes that for precision medicine, patient selection is key. Specifically, FDG PET/CT makes invisible PSMA-negative bone metastasis visible: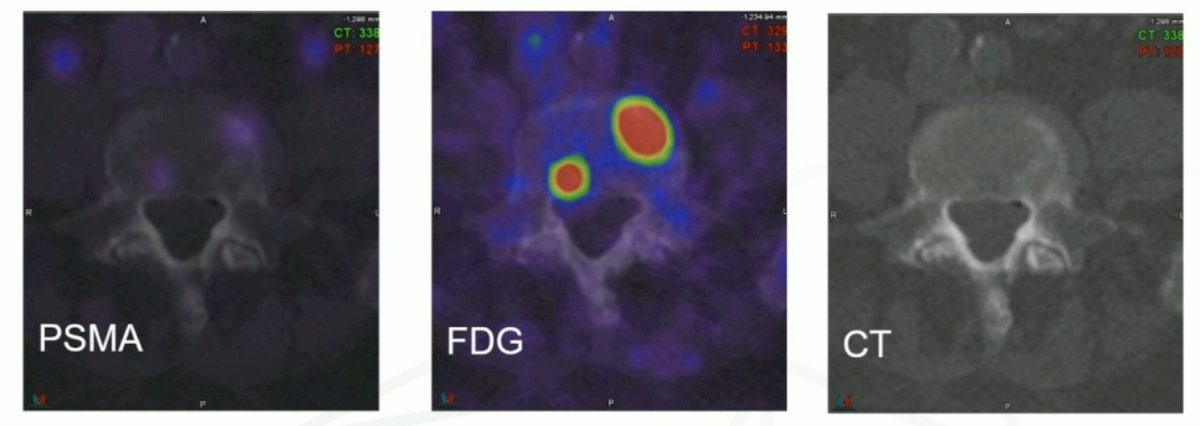
Quantitative PET is powerful, since the total tumor measure is superior to individual lesions, requiring automated reproducible solutions. PSMA SUVmean is a reasonable biomarker, as is 18F-FDG (superior to ECOG, ALP, hemoglobin, bone metastases, and liver metastases):4![Quantitative PET is powerful, since the total tumor measure is superior to individual lesions, requiring automated reproducible solutions. PSMA SUVmean is a reasonable biomarker, as is 18F-FDG [4] (superior to ECOG, ALP, hemoglobin, bone metastases, and liver metastases](/images/com-doc-importer/169-snmmi-2024/snmmi-2024-saul-hertz-s-theranostic-dream-is-shaping-the-future-of-cancer-care-bridging-evidence-based-medicine-and-precision-oncology/image-12.jpg)
Dr. Hofman highlighted that there have been >10 Australian PSMA theranostic trials, as highlighted in the following figure: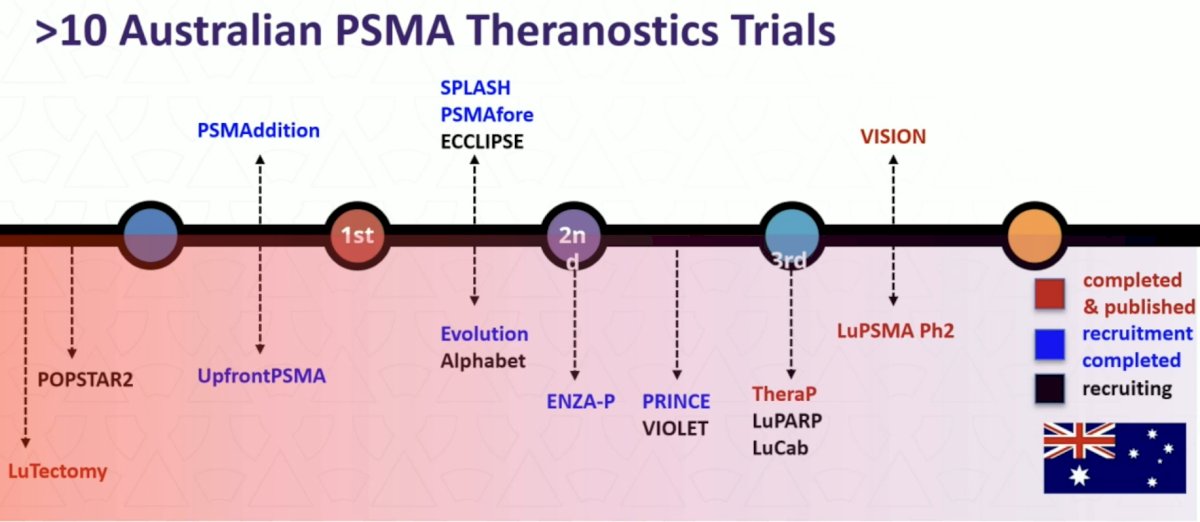
To conclude his presentation, Dr. Hofman highlighted several ongoing clinical trials. This included the UpFrontPSMA trial, which is a randomized phase 2 trial of 177Lu-PSMA-617 followed by docetaxel versus docetaxel alone for newly diagnosed high-volume mHSPC (NCT04343885):
Recruitment for this trial was completed in April 2013 and results are expected in Q3 of 2024. LuTectomy initial results were published in 2023.5 This is a single-center, single-arm, phase 1/2 study assessing the administration of 177Lu-PSMA-617 before radical prostatectomy in men with high-risk localized prostate cancer:![LuTectomy initial results were published in 2023 [5]. This is a single-center, single-arm, phase 1/2 study assessing the administration of 177Lu-PSMA-617 prior to radical prostatectomy in men with high-risk localized prostate cancer](/images/com-doc-importer/169-snmmi-2024/snmmi-2024-saul-hertz-s-theranostic-dream-is-shaping-the-future-of-cancer-care-bridging-evidence-based-medicine-and-precision-oncology/image-16.jpg)
This study included 20 patients, 10 in Cohort A (received one cycle of therapy) and 10 in Cohort B (received two cycles of therapy) followed by radical prostatectomy six weeks later. Overall, nine (45%) patients achieved >50% PSA decline, histopathological evidence of treatment effect was seen in 16 (80%) patients, one had minimal residual disease on final histology, and no patients achieved a complete pathological response. Additional ongoing trials are as follows:
- PRIMARY2: A phase 3 trial assessing the additive value of PSMA PET in men with negative or equivocal MRI to diagnose significant prostate cancer
- VIOLET: A phase 1/2 trial evaluation radioligand treatment in men with mCRPC with Terbium-161-PSMA-I&T
- AlphaBet: A phase 1/2 trial assessing Radium-223 in combination with 177Lu-PSMA-I&T in men with mCRPC
- LuCAB: A phase 1/2 trial assessing cabazitaxel in combination with 177Lu-PSMA-617 in mCRPC
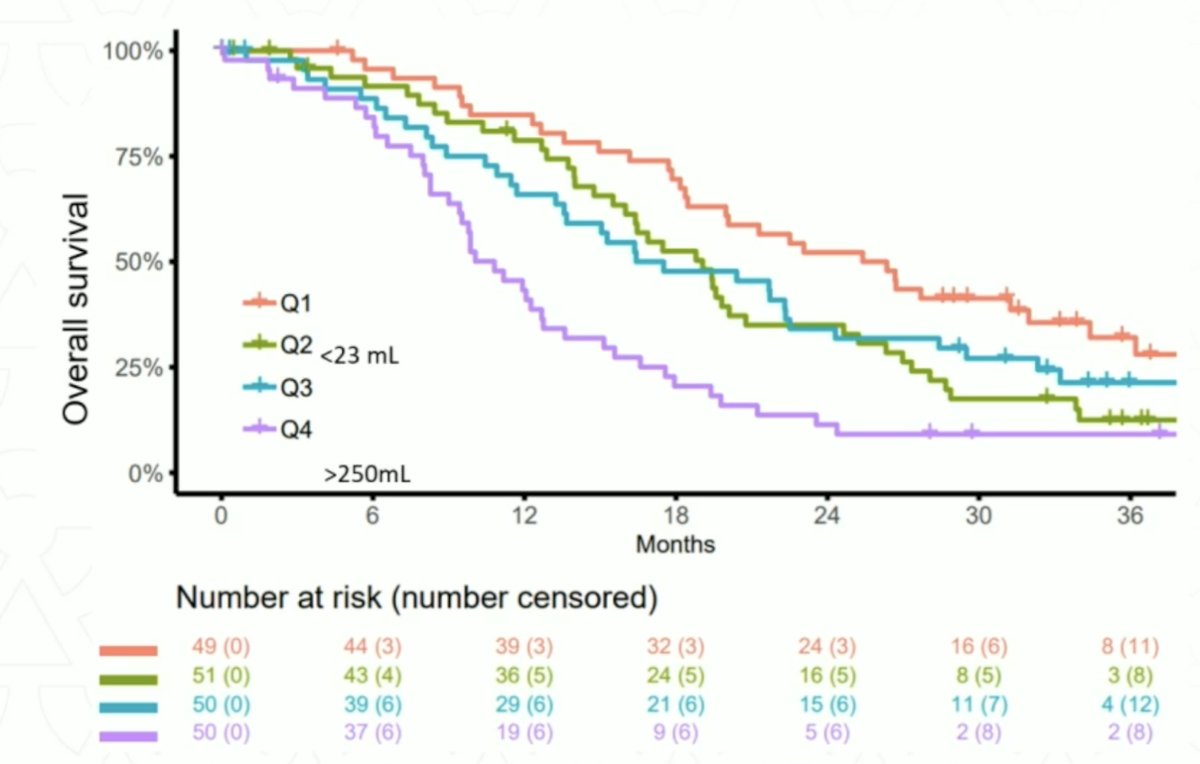
Dr. Hofman notes that the next big theranostic may be 68Ga-DPI-4452, a carbonic anhydrase IX-targeting peptide in patients with clear cell RCC.6 Among 3 patients, tumor uptake was observed at all time points (15 minutes to 4 hours), and across 36 lesions, the SUVmax at 1 hour after administration ranged from 6.8 to 211.6 (mean, 64.6). The kidneys, liver, and bone marrow demonstrated low activity, and [68Ga]Ga-DPI-4452 was rapidly eliminated from blood and urine: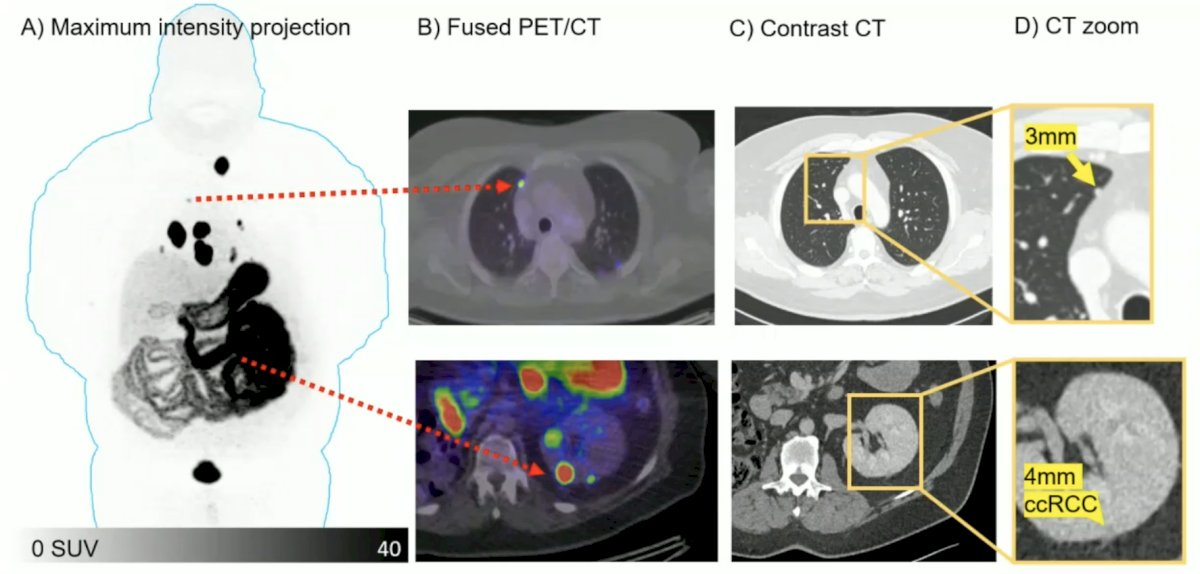
Dr. Hofman concluded his presentation discussing bridging evidence-based medicine and precision oncology by noting that the next phase of theranostics will require outstanding research, compassionate care, exceptional teaching, and phenomenal leadership.
Presented by: Professor Michael S. Hofman, MBBS (Hons), FRACP, FAANMS, FICIS, Peter MacCallum Cancer Center, University of Melbourne, Melbourne, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) Annual Meeting, Toronto, Ontario, Canada, Sat, June 8 – Tues, June 11, 2024.
References:
- Hofman MS, Lawrentschuk N, Francis, RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomized, multicentre study. Lancet 2020 Apr 11;395(10231):1208-1216.
- Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-center, single-arm phase 2 study. Lancet Oncol 2018 Jun;19(6):825-833.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Hofman MS, Emmett L, Sandhu S, et al. Overall survival with [177Lu]Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): Secondary outcomes of a randomized, open-label, phase 2 trial. Lancet Oncol. 2024 Jan;25(1):99-107.
- Eapen RS, Buteau JP, Jackson P, et al. Administering [177Lu]Lu-PSMA-617 Prior to Radical Prostatectomy in Men with High-risk Localized Prostate Cancer (LuTectomy): A Single-centre, single-arm, phase 1/2 study. Eur Urol. 2023 Oct 25:S0302-2838(23)03087-7.
- Hofman MS, Tran B, Feldman DR, et al. First-in-human safety, imaging, and dosimetry of a Carbonic Anhydrase IX-Targeting Peptide [68Ga]Ga-DPI-4452, in patients with clear cell renal cell carcinoma. J Nucl Med. 2024 Feb 22;65(5);740-743.


