(UroToday.com) The 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) annual meeting featured a session on prostate cancer, and a presentation by Dr. Nat Lenzo discussing preliminary results of ProstACT SELECT, specifically the safety, tolerability, and dosimetry of TLX591 with best standard of care in patients with PSMA-expressing metastatic castration resistant prostate cancer. PSMA has proven to be an ideal therapeutic target in prostate cancer. Monoclonal antibodies are distinguished by their internalization, long retention, and functional selectivity for tumor-expressed PSMA and can enable a short, patient-friendly dosing regimen with low occurrence of off-target side effects while delivering a meaningful therapeutic index. (177Lu) rosopatamab textraxetan (ie. TLX591) is a first-in-class radio-antibody drug conjugate investigational therapy that may be a potential treatment for prostate cancer, with previous clinical evidence supporting its favorable safety profile and specificity. TLX591 has been evaluated in more than 240 prostate cancer patients in eight phase 1/2 studies to date, with a 40+ month median overall survival in heavily pre-treated patients. Additionally, these studies demonstrated PSA response and dose-response profile for key measures of activity. Fractionated dosing manages hematologic safety while delivering a highly targeted and potent radiation dose to prostate cancer metastases.
At the SNNMI 2024 annual meeting, Dr. Lenzo and colleagues presented the preliminary results from ProstACT-SELECT (NCT04786847), a phase 1 study with the primary objective to determine whole body distribution, organ radiation, and assess the safety and tolerability of TLX591 administered with standard of care for patients with PSMA-expressing, metastatic castration-resistant prostate cancer progressing despite prior treatment with a novel androgen axis drug.
Participants received two intravenous infusions of TLX591 14 days apart. Cohort 1 (n = 5) received a 27mCi dose followed by 76mCi dose and cohort 2 (n = 23) received two doses of 76mCi (total cumulative dose of 152mCi). 68Ga-PSMA-11 PET imaging was performed to select patients, confirm baseline PSMA-positive lesions, and ensure TLX591 tumor targeting. For both cohorts, SPECT images and pharmacokinetic blood samples were acquired after both TLX591 infusions at the following time points: 4 hours, 24 hours, 96 hours, Day 7, and Day 13. Dosimetry analysis and qualitative comparison of biodistribution of tracer level of TLX591 were demonstrated by SPECT, and standard of care continued according to standard practice. The primary endpoints included:
- The absorbed radiation dose of administered TLX591 to kidneys, liver, lungs, spleen, bone/red marrow, and salivary glands
- Tumor-to-healthy tissue ratios and residence times
- Type, frequency, and severity of treatment-emergent adverse events
There were 28 participants included in this analysis:
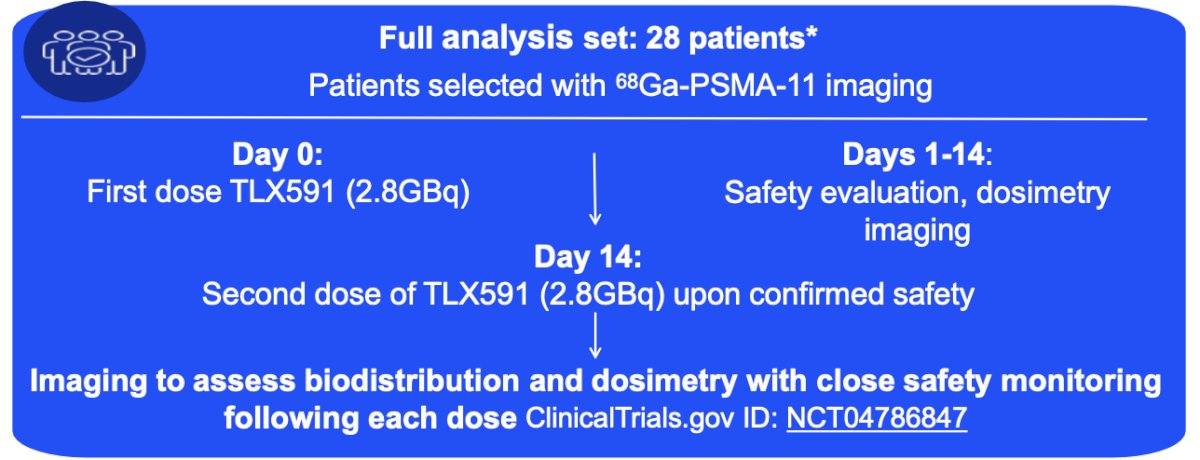
TLX591 and 68Ga-PSMA-11 uptake was consistent on SPECT imaging, within detection sensitivity and resolution limits. Radiation exposure to key organs was within prescribed safety limits. The highest absorbed dose was 13Gy in the liver, and the lowest dose was less than 1 Gy in salivary glands, with 3.5 Gy in kidneys. A retention period up to 14 days post-injection suggests internalization and the ability to efficiently deliver payload to tumors. No new safety signals were observed, and there were no non-hematologic adverse events Grade ≥ 3. Hematological adverse events included grade 3 thrombocytopenia (25%) and neutropenia (38%), which were in line with the profile expected for radio-antibody drug conjugate, as well as grade 4 thrombocytopenia (25%) and neutropenia (4%), which were transient. Overall, 64% of patients had a PSA reduction and median radiographic progression free survival was 8.8 months. The following is an example of a patient with metastatic castration resistant prostate cancer with high disease burden:

Dr. Lenzo concluded his presentation by discussing preliminary results of ProstACT SELECT with the following take home messages:
- TLX591 represents a first-in-class radio-antibody drug conjugate that demonstrates a favorable safety and tolerability profile administered in a patient-friendly dosing regimen of two cycles, 14 days apart
- The 68Ga-PSMA-11 imaging agent and TLX591 therapeutic agent are reaching the same target, an important consideration when evaluating a companion diagnostic for patient selection and monitoring
- Additionally, the liver absorbed dose was well below the 32 Gy limit, and there was minimal uptake in salivary glands
- Data from the SELECT study supports the potential benefits of an antibody-based approach and reinforces data published to date
- Investigation of TLX591 is continuing in the phase 3 ProstACT GLOBAL study, which is currently underway
Presented by: Nat Lenzo, MD, EMBA, FRACP, FAANMS, GAICD, GenesisCare, Perth, Western Australia, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024


