(UroToday.com) The 2024 Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting held in Toronto, ON between June 8 and June 11 was host to the session Novel Approaches and Combination Therapies; Pre-Targeting Approaches. Dr. Thomas Hope discussed the combination treatment of prostate cancer using Pembrolizumab with PSMA-based radioligands.
Dr Hope began his presentation by explaining how T-cell activation and checkpoint blockade. Fundamentally, the immune system orchestrates an attack on cells flagged by antigen-presenting cells (APC), employing an array of checkpoints—including but not limited to PD-1/PD-L1, CTLA4, and CD28.1
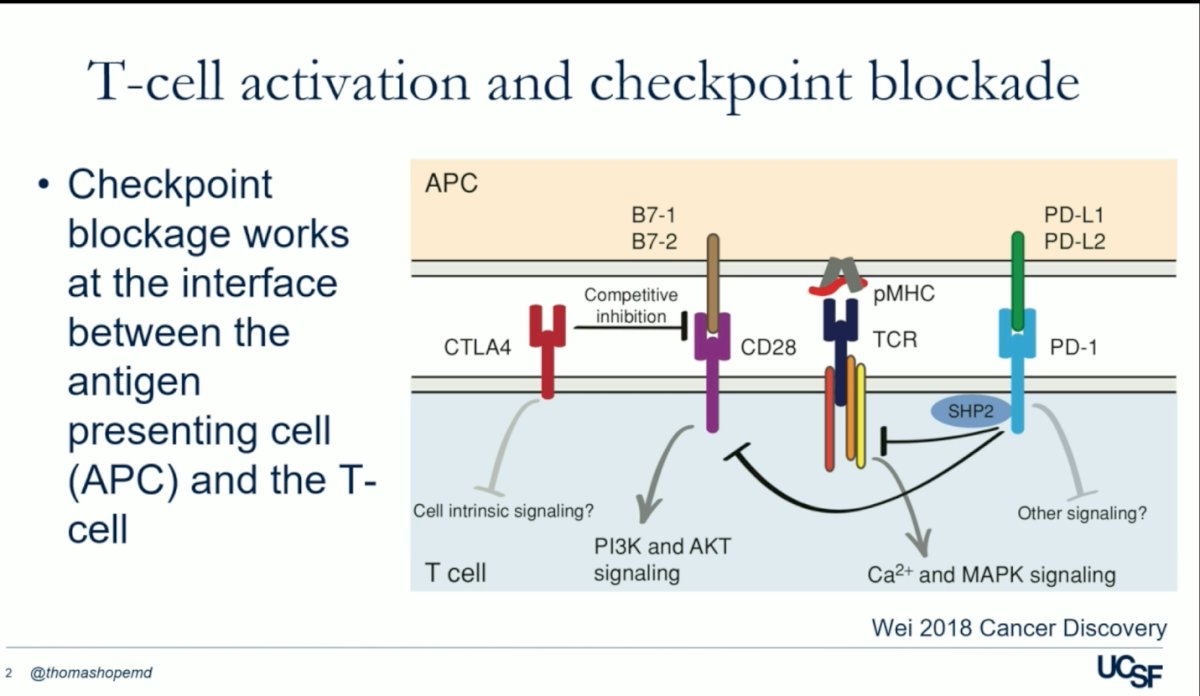
One of the pivotal checkpoints in immune regulation is the programmed cell death protein PD-1/PD-L1. PD-1, situated on T-cells, forms a binding interaction with PD-L1, thereby inhibiting the activation of T-cells by antigen-presenting cells (APCs). Immune checkpoint inhibitors (ICIs) like Pembrolizumab disrupt this binding and blockade removes inhibitory signals of T-cell activation, allowing T-cells to activate and effectively target aberrant cells, including cancer cells.1
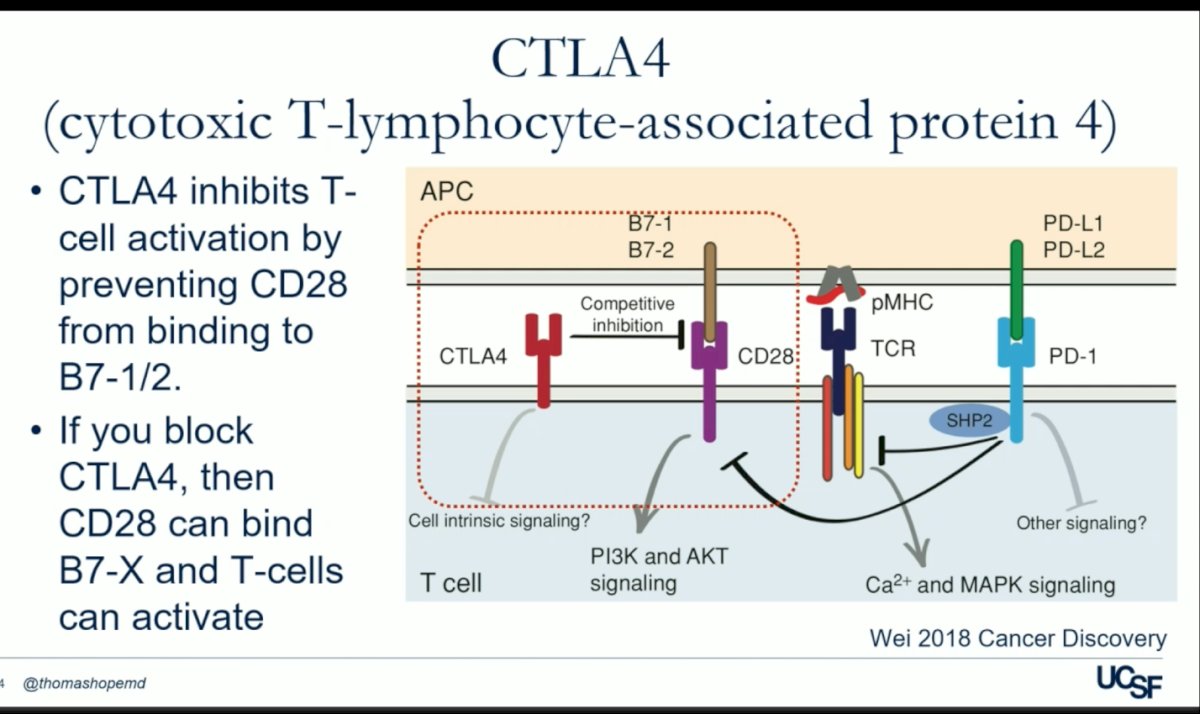
In a parallel mechanism, the cytotoxic T-lymphocyte-associated protein 4 (CTLA4) hinders T-cell activation by impeding the binding of CD-28 to B7 1/2 on the surface of antigen-presenting cells (APCs). By intercepting CTLA4, CD28 gains access to B7, facilitating T-cell activation, and subsequently enabling the immune system to target abnormal cells effectively. Ipilimumab is a CTLA-4 monoclonal antibody that stimulates the immune system. This medication would be also discuss in this presentsation, in combination with radioligant therapy.
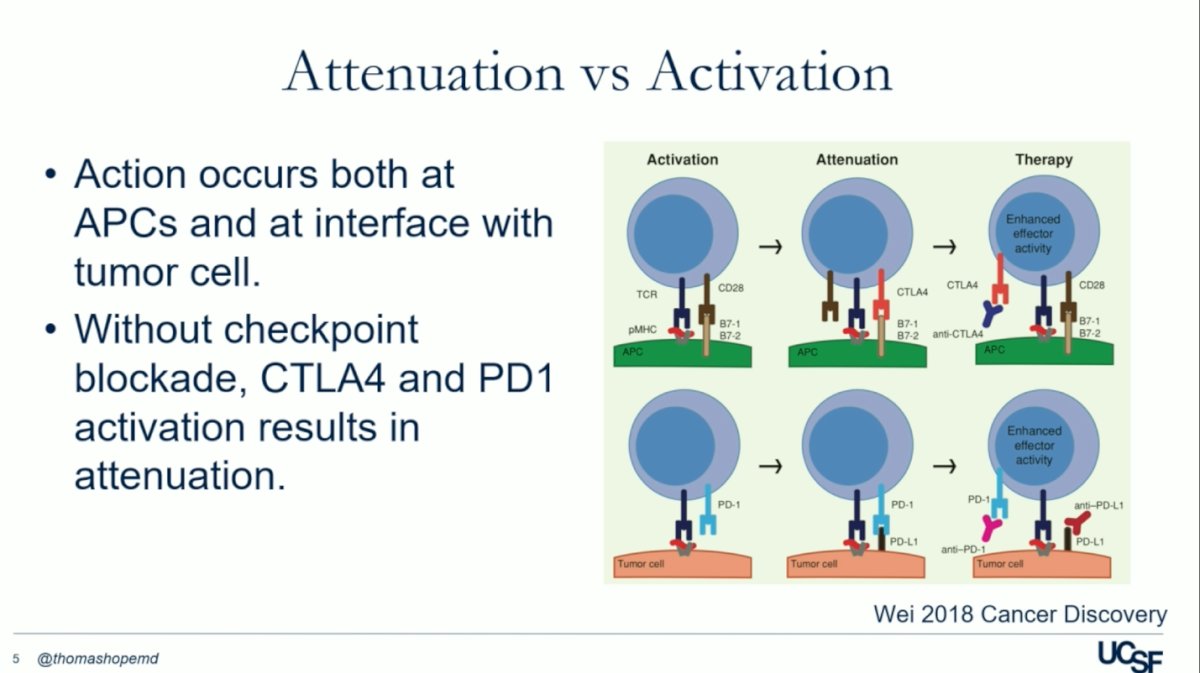
The molecular pathways of immune checkpoint inhibitors (ICIs) involve a sequence of events: T-cell activation, regulatory modulation (via PD1/PDL1 and CTLA4/CD28), and therapeutic intervention with anti-CTLA4 or anti–PD-1 antibodies. This intervention is crucial for lifting negative regulation. Without checkpoint blockade, CTLA4 and PD1 activation results in attenuation, as illustrated in the figure below. Furthermore, ICIs may also work through mechanisms like Treg depletion, bolstering T-cell stimulation within the tumor microenvironment, blocking PD-L1 signals from non-tumor cells, and inhibiting PD-L1/B7-1 interactions.1
The adoption of immune checkpoint inhibitors (ICI) has markedly increased, fostering greater assurance in their usage across diverse cancer types. Dr. Hope shared a compelling example regarding melanoma: Nivolumab demonstrates remarkable efficacy, especially in BRAF-negative cases, leading to a notable 58% enhancement in overall survival—an outcome previously unseen in this context prior to the approval of ICI.

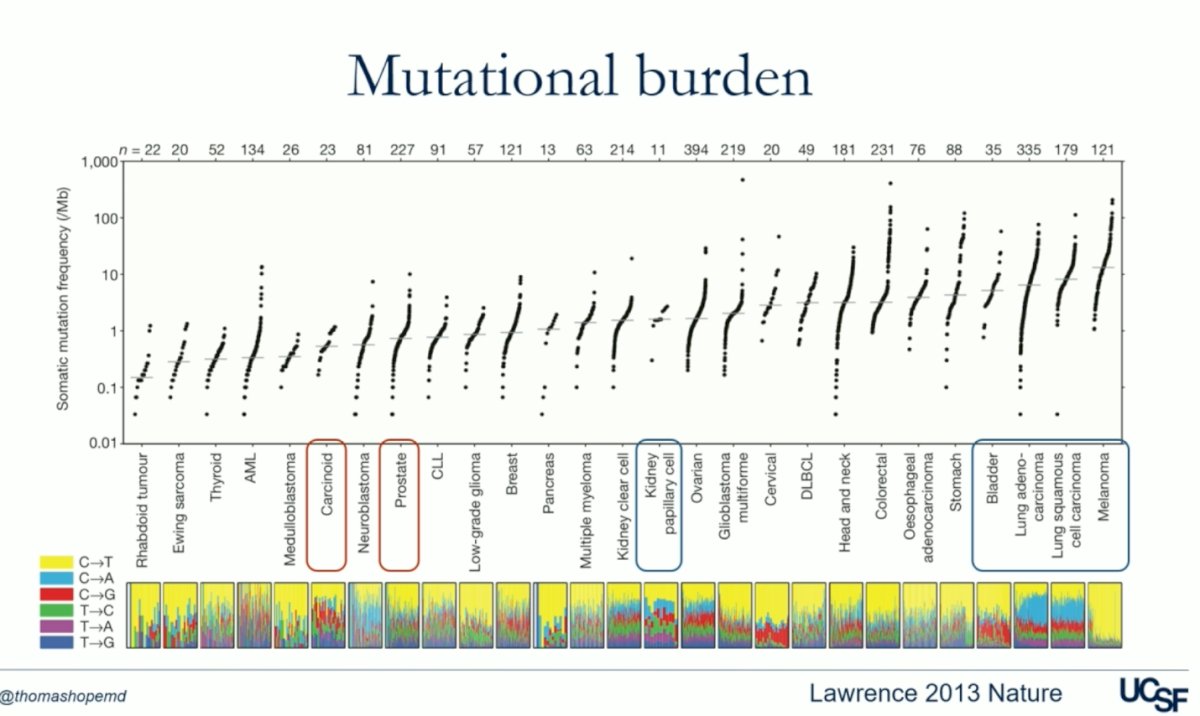
One key mechanism behind the outstanding response to ICI in melanoma is related to the mutational burden. For example, in the figure below, bladder cancer, lung adenocarcinoma, squamous cell carcinoma, and melanoma have a very high mutational burden, which correlates with the significant response these tumors have had to ICI. In contrast, tumors such as neuroendocrine tumors and prostate cancer have a low mutational burden, leading to a less effective response to ICI.
The rationale behind combining ICI with radioligand therapy in prostate cancer is that targeted radioligand therapy might theoretically enhance the effectiveness of ICI. It could do so by enhancing the priming of an immune response or resetting the immunosuppressive (cold) tumor microenvironment in prostate cancer to boost effector function, as well by increasing the mutational burden which would theoretically improve the response to ICI.3
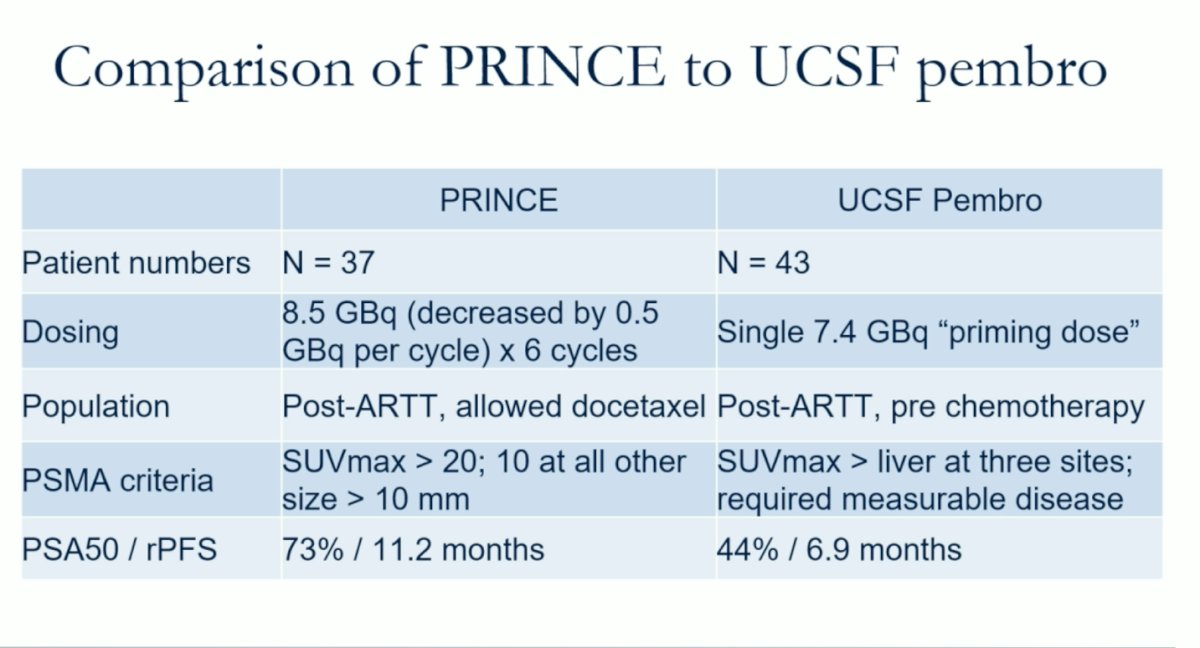
The PRINCE trial (NCT03658447) included metastatic castration-resistant prostate cancer (mCRPC) patients with high PSMA expression and no discordant FDG avid disease who had been treated with androgen receptor pathway inhibitors (ARPIs) and docetaxel (73%). They enrolled 37 patients who received up to 6 cycles of Lu-PSMA-617 every 6 weeks and 200 mg of pembrolizumab every 3 weeks for up to 2 years. The patients received a median of 5 cycles (around 6 months of treatment). The PSA50 response was 76%, with 78% of patients with measurable disease achieving a partial response. The median PSA progression-free survival (PFS) was 8.2 months, and the median radiological PFS (rPFS) was 11.2 months. At 24 weeks, the rPFS was 68%. The median OS was 18 months.2 The combination therapy demonstrated promising activity with manageable toxicities and set the stage for the University of California, San Francisco (UCSF) trial discussed below.

he UCSF trial (NCT03805594) was an open-label, dose-expansion, phase 1 study. It included men aged 18 years or older with progressive mCRPC and an Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0 or 1, who had progression on one or more ARPIs, had at least three PSMA-avid lesions on 68Ga-PSMA-11 PET and have not been treated with prior chemotherapy. The study was divided into part A and part B: in part A, patients received a single dose of 177Lu-PSMA-617 (7.4 GBq) intravenously either 28 days before (schedule 1), concomitant with (schedule 2), or 21 days after (schedule 3) the start of pembrolizumab (200 mg every 3 weeks). The primary endpoint of part A was determining the phase 2 schedule for part B. Part B enrolled 25 patients using the recommended phase 2 schedule and aimed to report the objective response rate. Dr Hope highlighted that one of the main differences between the UCSF and the PRINCE trials, is that the UCSF trial used a single dose of 177Lu-PSMA-617 aiming to prime the immune system before the first dose of Pembrolizumab.
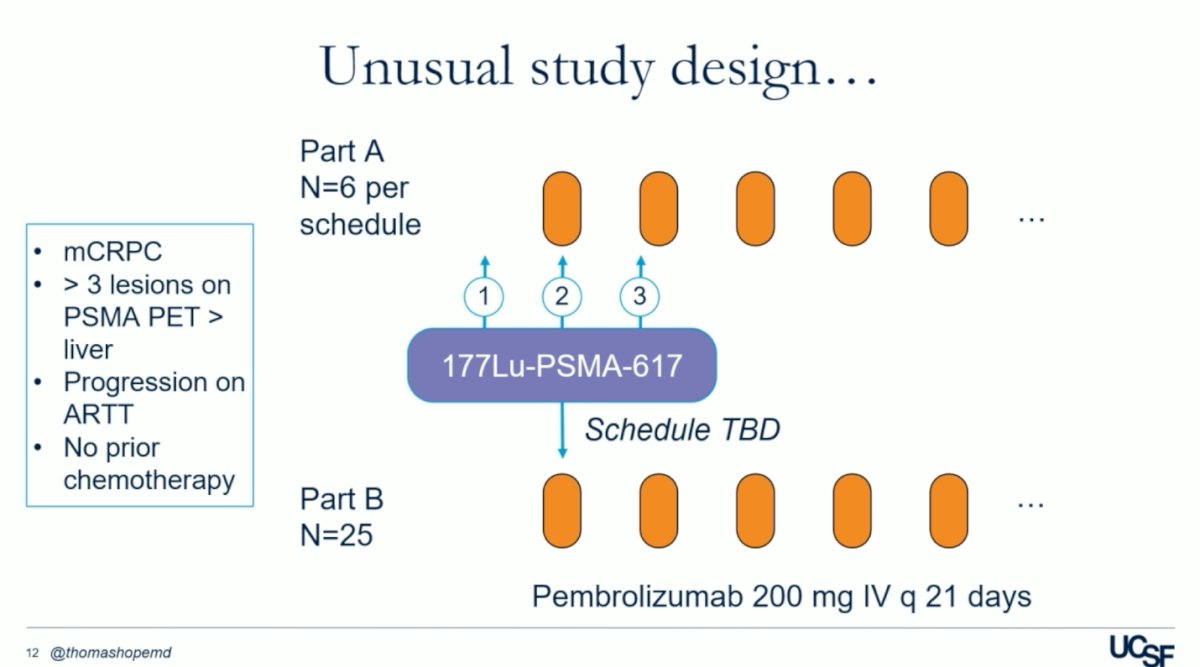
Dr. Hope explained that the rationale behind using a single priming dose of 177Lu-PSMA-617 was to prevent immune ablation with repeated Lutetium dosing at fixed intervals. This approach aimed to extend the response with pembrolizumab while minimizing hematologic and other cumulative toxicities associated with Lutetium dosing. By avoiding excessive Lutetium exposure upfront, patients could potentially receive subsequent Lutetium dosing in later stages of the disease, thereby maximizing treatment effectiveness while managing toxicity levels.
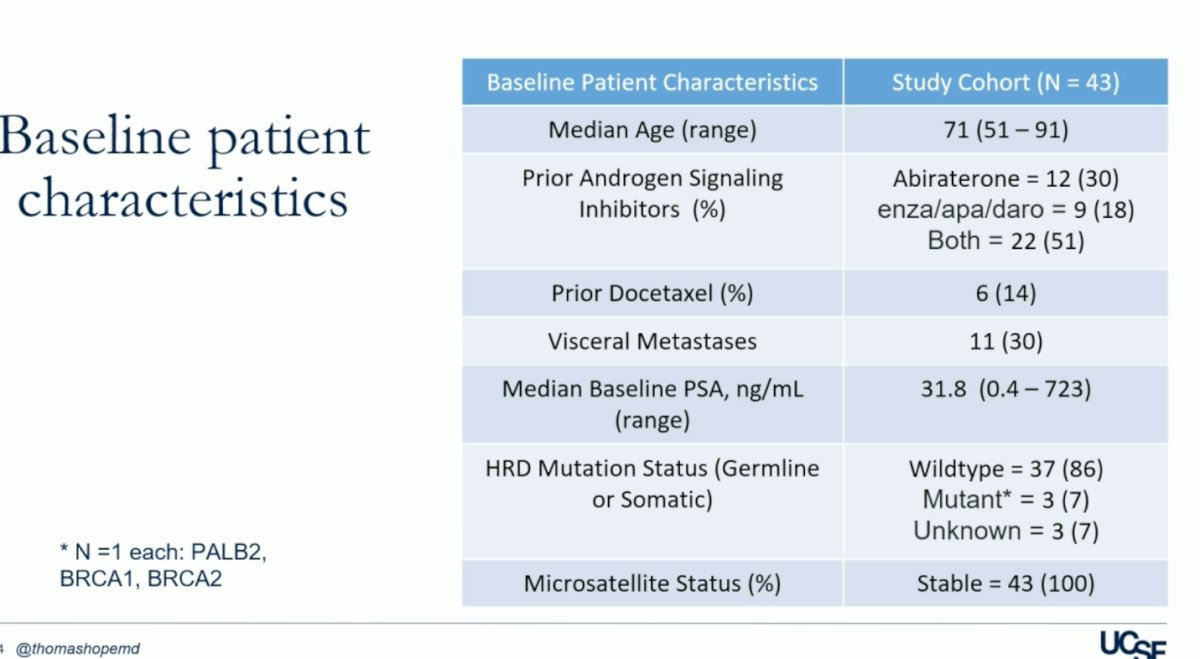
A total of 43 patients were enrolled in the trial, with 18 in part A and 25 in part B. Baseline patient characteristics are summarized in the table below. Of note, only 14% of the patients received prior Docetaxel, 30% had visceral metastases, and all patients had microsatellite instability (MSI).
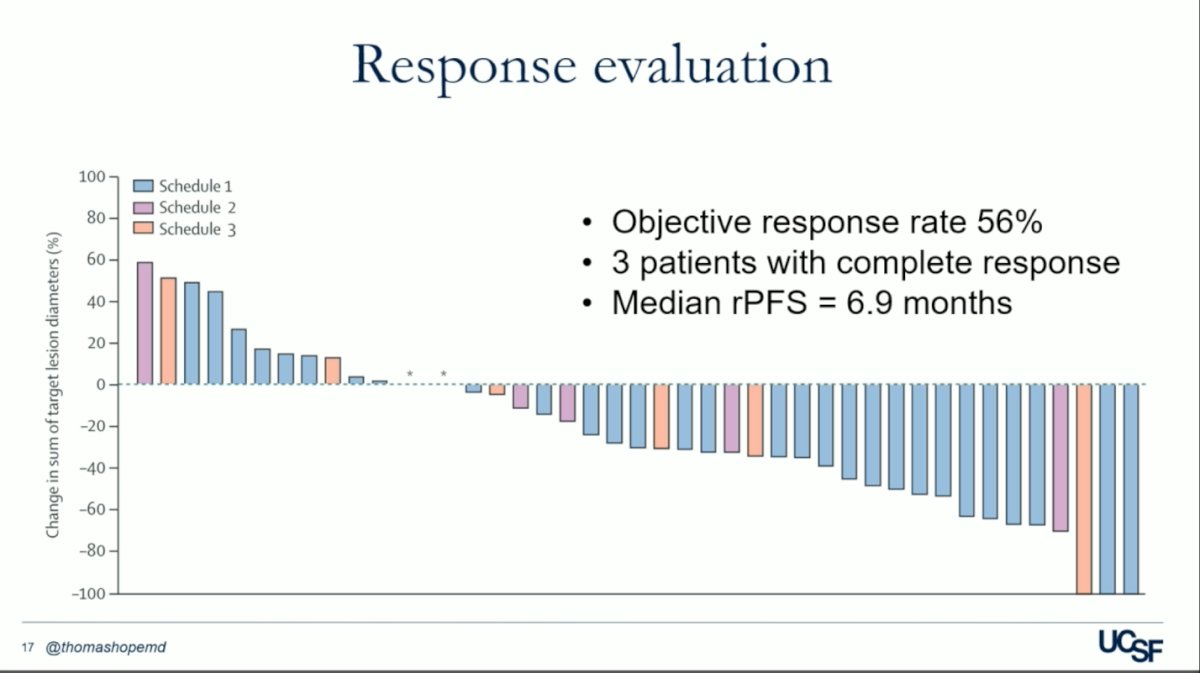
The median follow-up was 16.5 months. Schedule 1 was selected as the recommended phase 2 schedule based on safety and feasibility. In part B, 56% of patients had a confirmed objective response, 3 patients achieved a complete response and the median rPFS was 7 months.
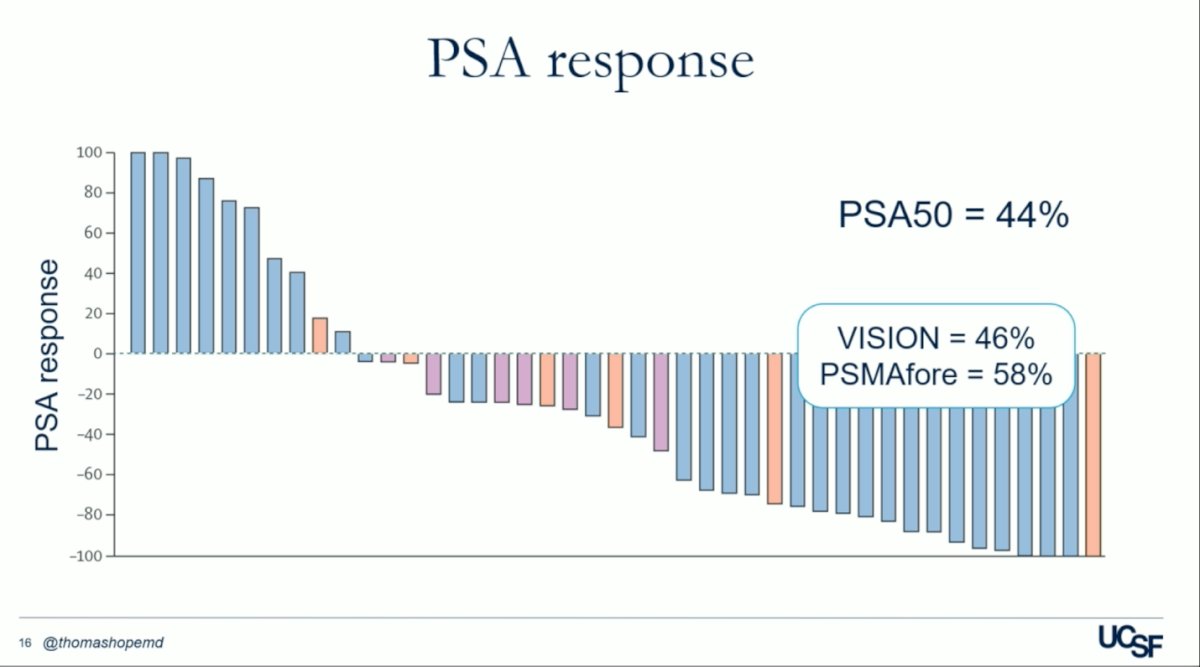
The PSA50 response in the UCSF trial was nearly identical to that of the VISION trial, with rates of 44% and 46%, respectively.4 Dr. Hope highlighted this similarity, raising the question of whether more doses of 177Lu-PSMA-617 are necessary given the promising PSA response achieved with just a single priming dose.
The progression-free survival (PFS) in the UCSF trial was 7 months, compared to 8.7 months in the VISION trial, as illustrated in the figure below.
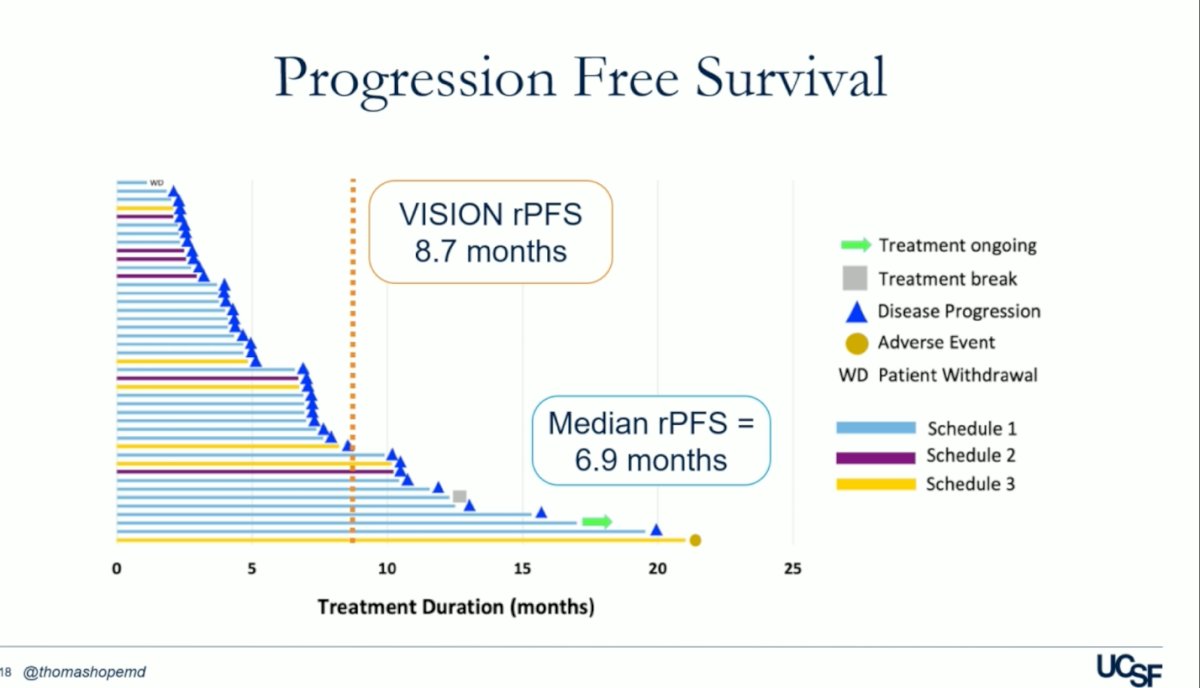
Out of the 43 patients, only two (2/43) experienced treatment-related adverse events (TRAEs) of grade 3 or worse, which included inflammatory arthritis and pneumonitis. No TRAEs of grade 4 or 5 were documented, and there were no treatment-related deaths.
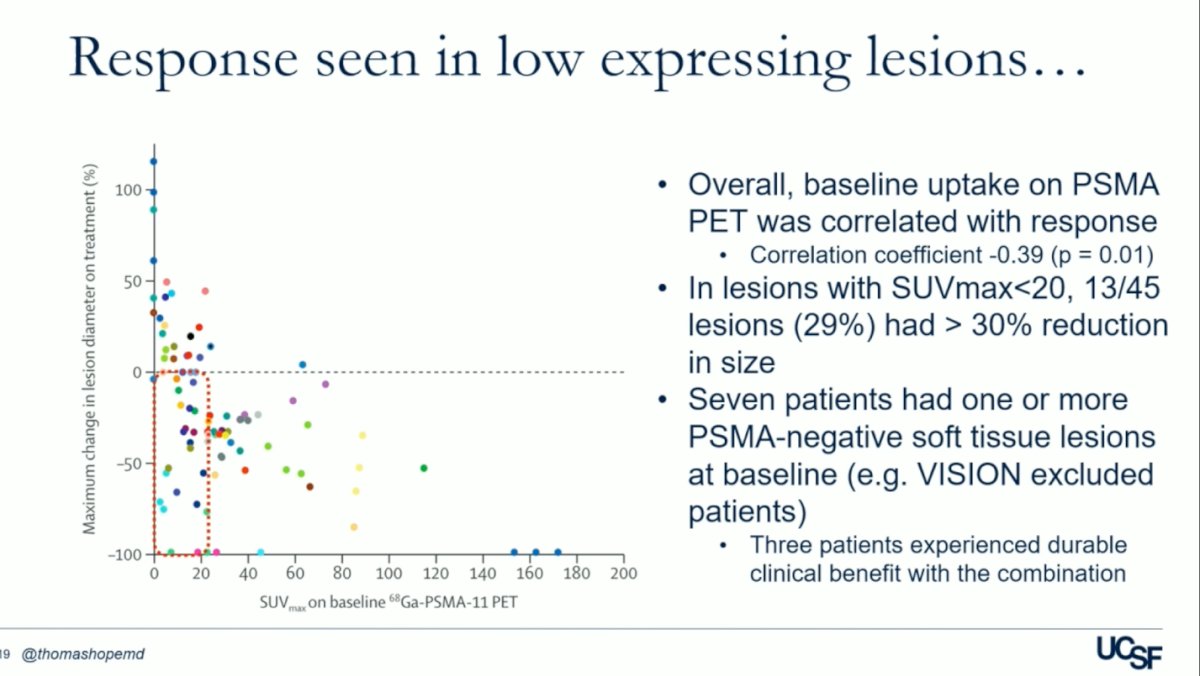
Dr. Hope discussed that for this phase 1 trial, they allowed patients with lower PSMA uptakes in the liver to be enrolled. They observed that overall, baseline uptake on PSMA PET correlated with response (p = 0.01). Additionally, in lesions with SUVmax<20, 13 out of 45 lesions (29%) experienced a >30% reduction in size. Notably, in the UCSF trial, seven patients had one or more PSMA-negative soft tissue lesions at baseline, a criterion that would have excluded them from the VISION trial. The maximum change in lesion diameter on treatment according to the SUVmax on baseline PSMA-PET is illustrated below.
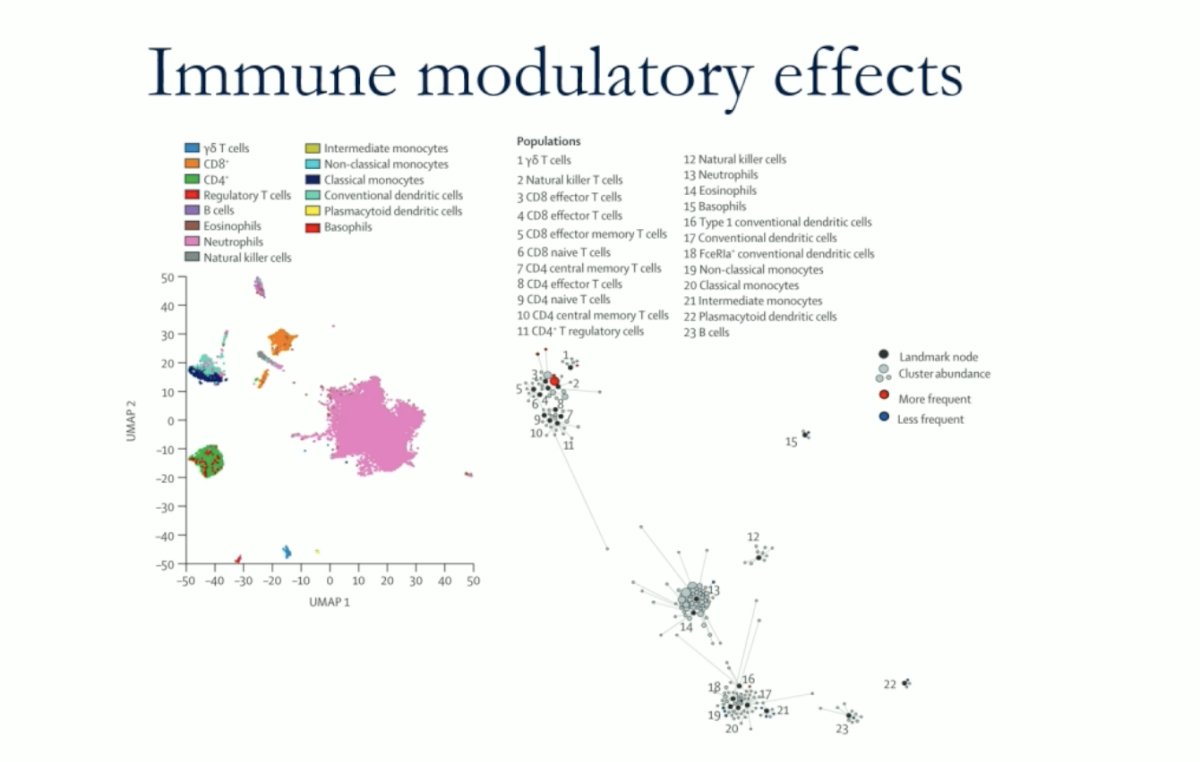
An exploratory analysis of circulating mononuclear cell subsets using mass cytometry (CyTOF) analysis showed that pembrolizumab reduced PD-1 staining in T-cell populations and they found multiple subtypes of activated cells as reflected below.

In their analysis, they compared the relationships between cell clusters identified in samples obtained from patients with a response and those who had no response to treatment, aiming to determine which cells were upregulated or downregulated. They found that T cells were upregulated. Specifically, at all post-treatment time points, patients who had an objective response showed higher cell cluster frequencies of CD8+ effector cells, CD8+ effector memory cells, γδT cells, and natural killer T cells compared to non-responders.
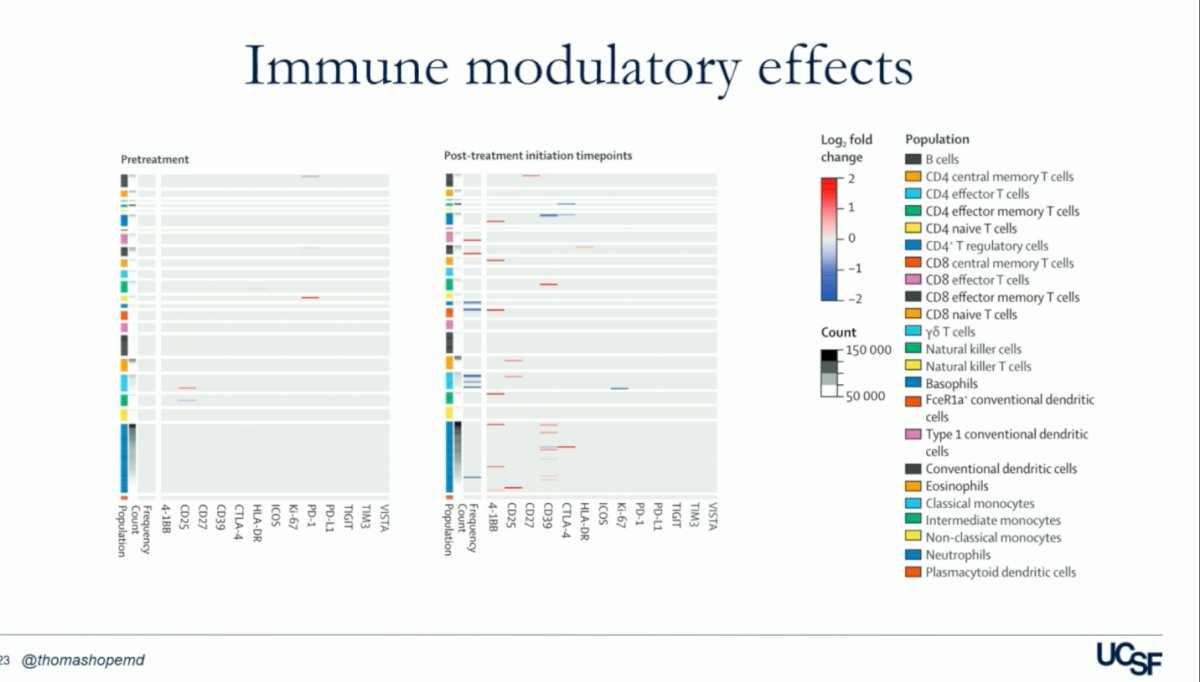
Patients who achieved an objective response exhibited lower cell cluster frequencies of basophils, FceR1a+ conventional dendritic cells, classical monocytes, neutrophils, and plasmacytoid dendritic cells compared to non-responders. This observation underscores the importance of priming the immune system with the single dose of Lutetium. However, while they couldn't conclusively demonstrate the benefit of immune priming, their findings suggest that the immune response plays a crucial role. It remains uncertain whether checkpoint inhibitions amplify these responses. The heatmap below summarizes the log2-fold changes resulting from statistical scaffold analysis of cell cluster frequency and functional markers.
Dr. Hope compared the UCSF study with the PRINCE trial, both single-arm studies, utilizing Pembrolizumab in addition to radioligand therapy with Lutetium-PSMA in mCRPC patients. The table below summarizes the main differences.
The EVOLUTION trial (NCT05150236) is an open label, randomized, multicenter phase 2 clinical trial aiming to recruit 110 participants. Patients will be randomized in a 2:1 ratio to receive either 6 cycles of 177Lu-PSMA in combination with Ipilimumab and Nivolumab or 177Lu-PSMA alone. Stratification will be based on prior exposure to docetaxel.
UCSF aims to enroll 48 patients in their phase 2 trial, which involves repeated Lu-PSMA dosing at PSA-defined intervals. This single-arm study will administer Pembrolizumab with 6 cycles of 177Lu-PSMA, utilizing an adaptive dosing approach. The dosing of 177Lu-PSMA will be determined by PSA response, with subsequent doses given at PSA progression, defined as either a PSA rise of 25% or a rise of 2 ng/dL. Enrollment began in January 2024, and to date, they have enrolled 9 patients.
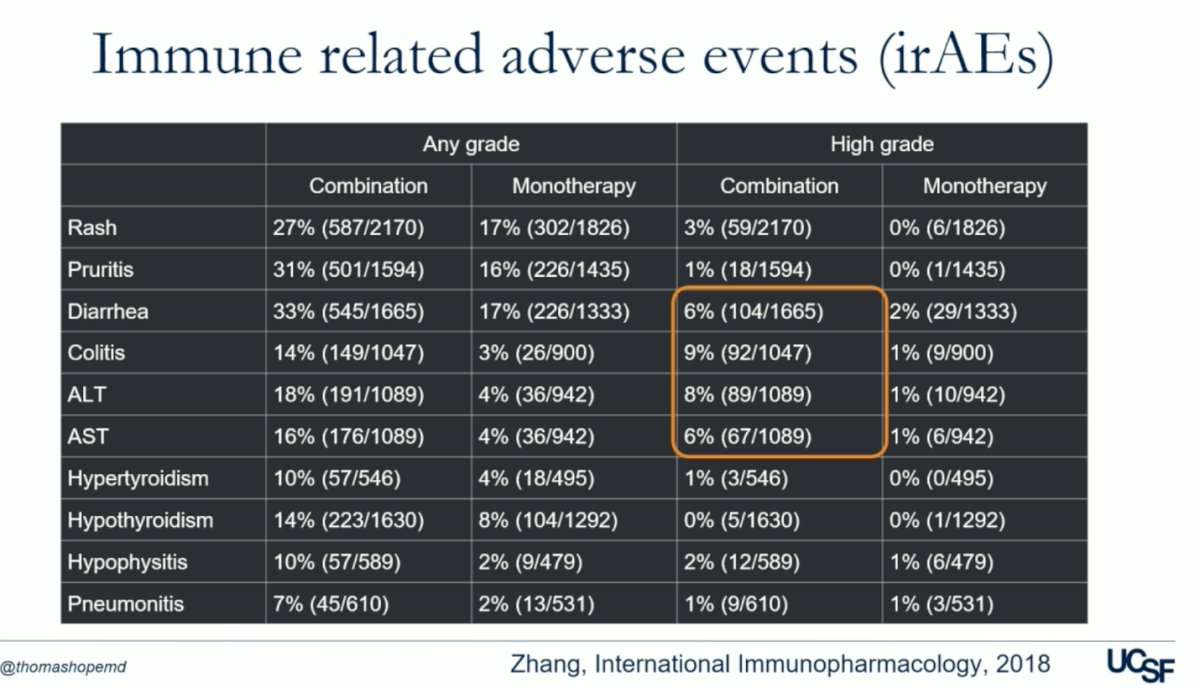
The advantage of UCSF's approach lies in the adaptive dosing strategy based on PSA response, which allows for personalized treatment tailored to individual patient needs. Aiming to optimize treatment efficacy while minimizing the risk of toxicity associated with repeated dosing. Additionally, this approach addresses concerns raised about the combination of ICI-ICI therapies like Nivolumab/ipilimumab, which have been associated with higher rates of immune-related adverse events (IRAEs) compared to ICI monotherapy. Indeed, the table below demonstrates that high-grade treatment irAEs are are nearly three times more frequent with ICI-ICI combination therapy.5
Dr Hope concluded his presentation by delivering the following key messages:
- There have been two trials combining pembrolizumab with PSMA-radioligand therapy (PRINCE and the UCSF study)
- Results are promising but unclear if the combination is additive or if the radioligand therapy is driving the response
- Two ongoing trials will help answer this question: EVOLUTION (NCT05150236) combining Ipilimumab/Nivolumab, and the UCSF phase 2 study with the adaptive-dosing approach
Presented by: Thomas Hope, MD, Vice Chair of Clinical Operations and Strategy in the Department of Radiology at University of California, San Francisco (UCSF) and Chief of Nuclear Medicine at the San Francisco VA Medical Center
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024
References:
- Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018 Sep;8(9):1069-1086. doi: 10.1158/2159-8290.CD-18-0367. Epub 2018 Aug 16. PMID: 30115704.
- Sandhu S, Joshua A, Emmett L, et al.. 577o prince: interim analysis of the phase IB study of 177LU-PSMA-617 in combination with pembrolizumab for metastatic castration resistant prostate cancer (mcrpc). Ann Oncol. 2021;32(suppl_5):S626-S627. 10.1016/j.annonc.2021.08.1090.
- Aggarwal R, Starzinski S, de Kouchkovsky I, Koshkin V, Bose R, Chou J, Desai A, Kwon D, Kaushal S, Trihy L, Rastogi M, Ippisch R, Aslam M, Friedlander T, Feng F, Oh D, Cheung A, Small E, Evans M, Fong L, Hope TA. Single-dose 177Lu-PSMA-617 followed by maintenance pembrolizumab in patients with metastatic castration-resistant prostate cancer: an open-label, dose-expansion, phase 1 trial. Lancet Oncol. 2023 Nov;24(11):1266-1276. doi: 10.1016/S1470-2045(23)00451-5. PMID: 37922930; PMCID: PMC10667020.
- Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, Tagawa ST, Nordquist LT, Vaishampayan N, El-Haddad G, Park CH, Beer TM, Armour A, Pérez-Contreras WJ, DeSilvio M, Kpamegan E, Gericke G, Messmann RA, Morris MJ, Krause BJ; VISION Investigators. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103. doi: 10.1056/NEJMoa2107322. Epub 2021 Jun 23. PMID: 34161051; PMCID: PMC8446332.
- Zhang B, Nie W, Han B. Immune-Related Adverse Events and Efficacy—The More It Hurts, the Better It Works? JAMA Oncol. 2021;7(6):944–945. doi:10.1001/jamaoncol.2021.0729


