(UroToday.com) The 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) annual meeting featured a session on prostate cancer, and a presentation by Dr. Jiarou Wang discussing preclinical and first-in-human studies of dansylated amino acid-modified long-acting PSMA derivative 68Ga/177Lu-LNC1011. 177Lu-PSMA-617 has inadequate uptake and retention in tumors and poor diagnostic efficacy:

177Lu-LNC1011 (177Lu-D-Dan-Phe-PSMA) is a novel long-circulating PSMA therapeutic probe whose structure is based on an albumin binder dansyl group. This study represents the first in human investigation, aiming to explore its maximum tolerated dose, safety, dosimetry, and initial treatment efficacy of 177Lu-LNC1011 in patients with metastatic castration-resistant prostate cancer.
This study employed an open-label, non-randomized design, representing the first human trial of its kind. It adopted a dose-escalation approach, enrolling metastatic castration-resistant prostate cancer patients who have passed pre-screening PSMA PET/CT. The treatment initiation occurred at a dose of 1.85 GBq over a 6-week period. Subsequent cohorts experienced sequential 50% dose escalations until the observation of dose-limiting toxicity: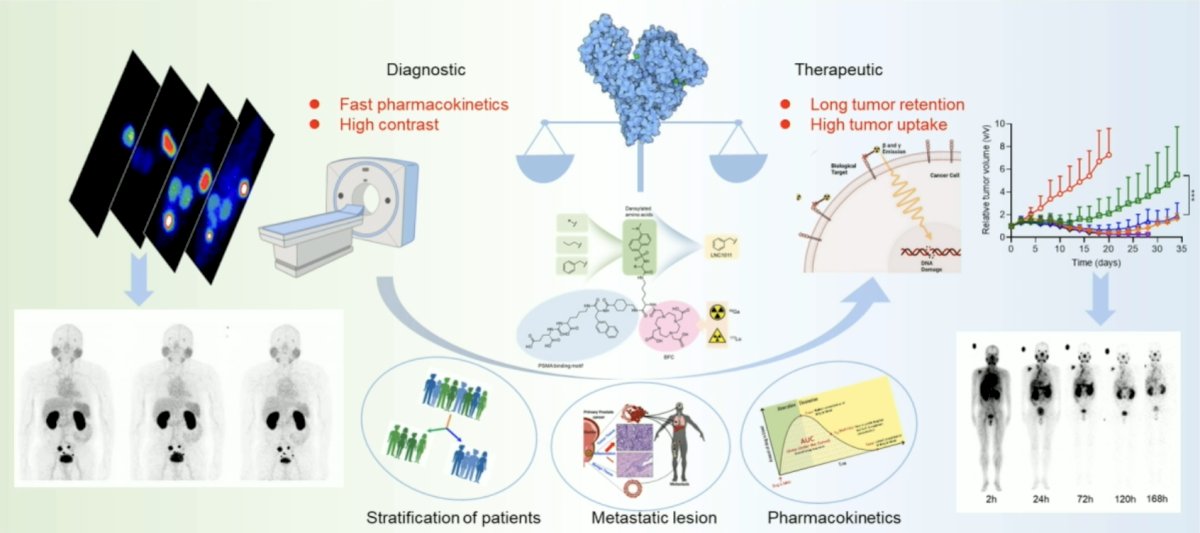
There were a total of 12 metastatic castration-resistant prostate cancer patients that received 177Lu-LNC1011 at doses ranging from 1.85 GBq to 3.7 GBq. No life-threatening adverse events were observed during the dosing observation period. Following two treatment cycles, dose level 1 and level 2 groups exhibited no hematologic toxicity, dose level 3 group showed Grade 1 thrombocytopenia. Effective dose for total-body was 0.10 ± 0.02 mSv/MBq, and the kidneys received the highest expected radiation dose, calculated to be 3.40 ± 0.98 mSv/MBq. Effective doses for salivary glands and red bone marrow were 1.54 ± 1.00 mSv/MBq and 0.11 ± 0.03 mSv/MBq, respectively. The average effective dose for tumors was 0.13 ± 0.02 mSv/MBq. According to the guidelines of the Prostate Cancer Clinical Trials Working Group-3, PSA response in dose level 1 group showed stable disease (66.67%) and partial response (16.67%), in dose level 2 group showed stable disease (66.67%), and in dose level 3 group showed stable disease (66.7%) and partial response (66.7%):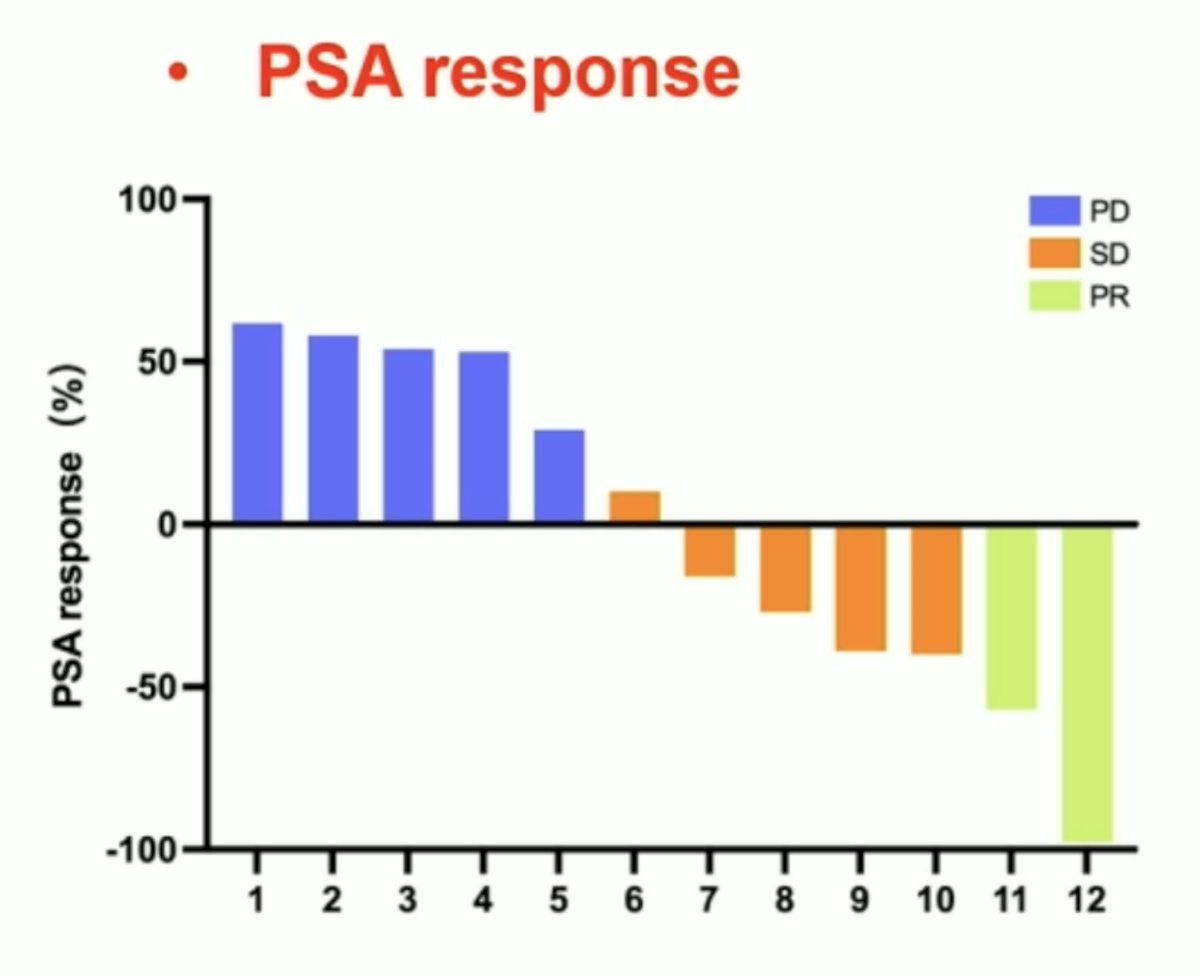
In comparison to PSMA-617, LNC1011 had similar salivary expected dose (1.54 ± 1.00 versus 1.25 ± 0.51) while demonstrating a notably higher effective dose (0.13 ± 0.02 versus 0.02 ± 0.003). When compared to an earlier long-acting PSMA formula EB-PSMA, which was based on Evans blue as an albumin binder, LNC1011 showcases significantly lower salivary dose (1.54 ± 1.00 versus 6.14 ± 1.40) but maintained a comparable tumor-effective dose (0.13 ± 0.02 versus 0.13 ± 0.04).
The following is representative whole-body anterior projective images taken at multiple time points within one week for an 80-year-old male with metastatic castration-resistant prostate cancer who received 177Lu-LNC1011 at a dose of 2.78 GBq. Tumor uptake remained high at 168 hours: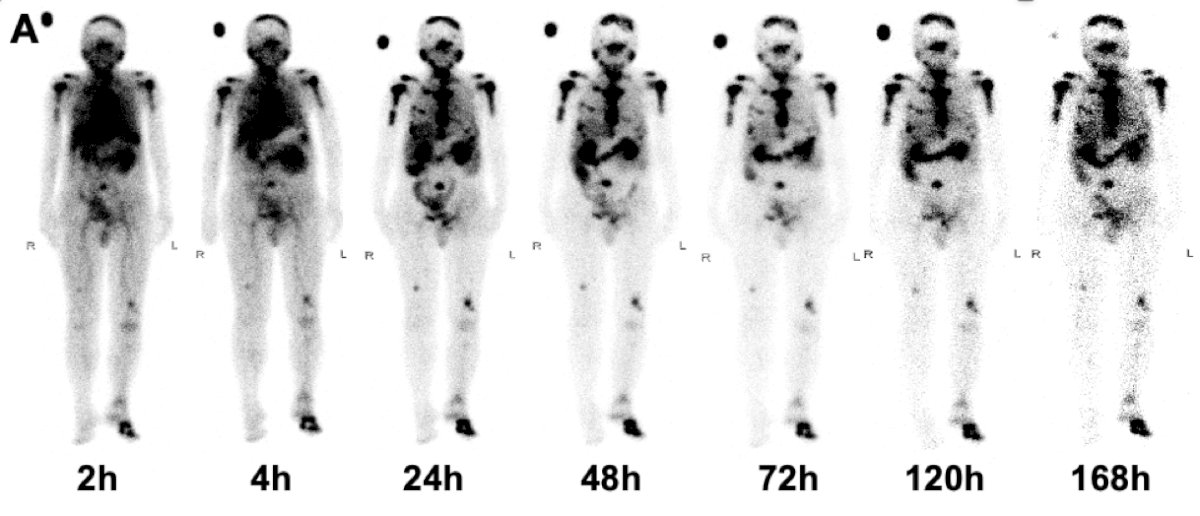
The estimated effective dose of 177Lu-LNC1011 compared to 177Lu-EB-PSMA and 177Lu-PSMA-617 exhibits remarkably low salivary glad uptake and a higher tumor effective dose: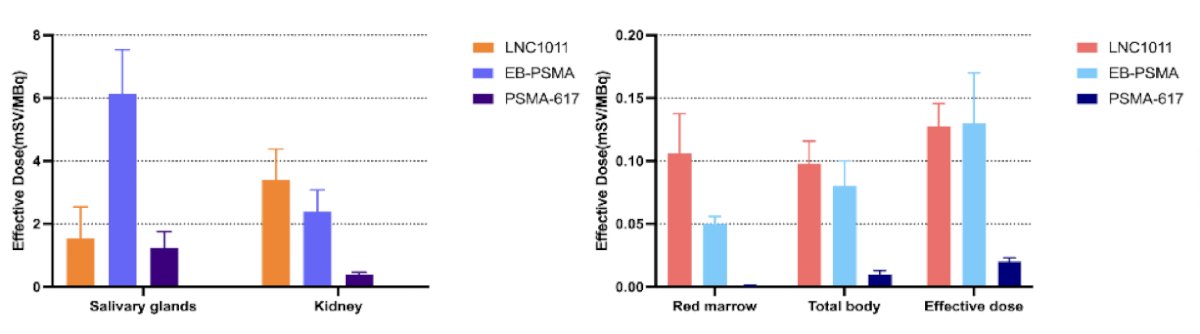
The following is a 65-year-old male patient after undergoing one cycle of 177Lu-LNC1011 treatment with a dosage of 3.7 GBq who exhibited a remarkable therapeutic response: the PSA level decreased from 21.1 to 0.52 ng/mL, and the SUVmax of the left scapular lesion decreased from 78.24 to 10.4, in addition to a reduction in the mediastinal and retroperitoneal lymph nodes: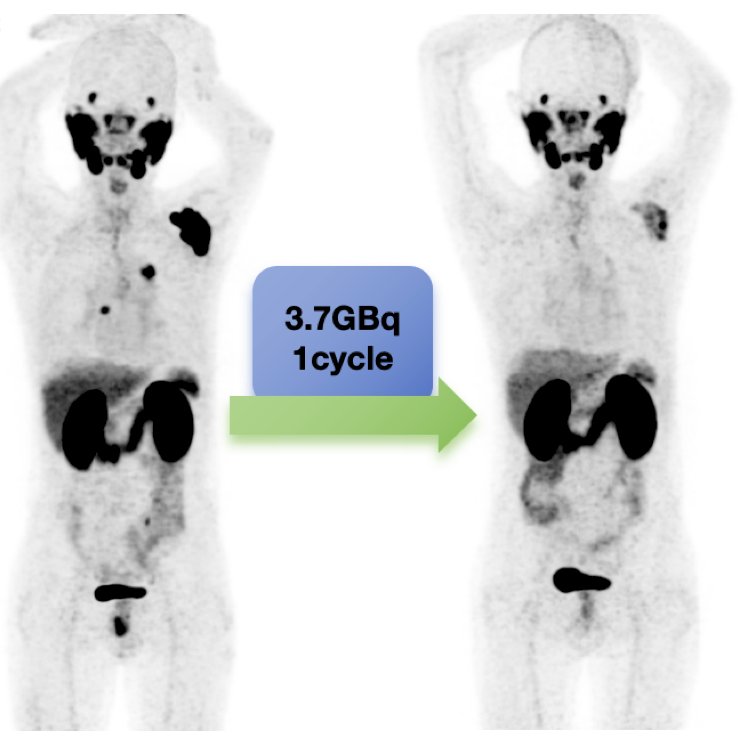
Dr. Wang concluded this presentation discussing preclinical and first-in-human studies of dansylated amino acid-modified long-acting PSMA derivative 68Ga/177Lu-LNC1011 with the following take-home messages:
- Preclinical study of 68Ga/177Lu-LNC1011 demonstrated significant improvement in tumor uptake and timely clearance from background organs
- 68Ga-LNC1011 in humans showed good diagnostic efficacy
- Dosimetry analysis revealed low uptake in the salivary glands and achieves a high tumor-absorbed dose
- The administration of 177Lu-LNC1011 at a dose of 3.7 GBq in the treatment of metastatic castration-resistant prostate cancer patients is well tolerated and has minimal side effects
- The therapeutic performance is promising
- 177Lu-LNC1011 is a promising candidate for a single-molecule theranostic ligand
Presented by: Jiarou Wang, MD, Peking Union Medical College Hospital, Beijing, China
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) Annual Meeting, Toronto, Ontario, Canada, Sat, June 8 – Tues, June 11, 2024.


