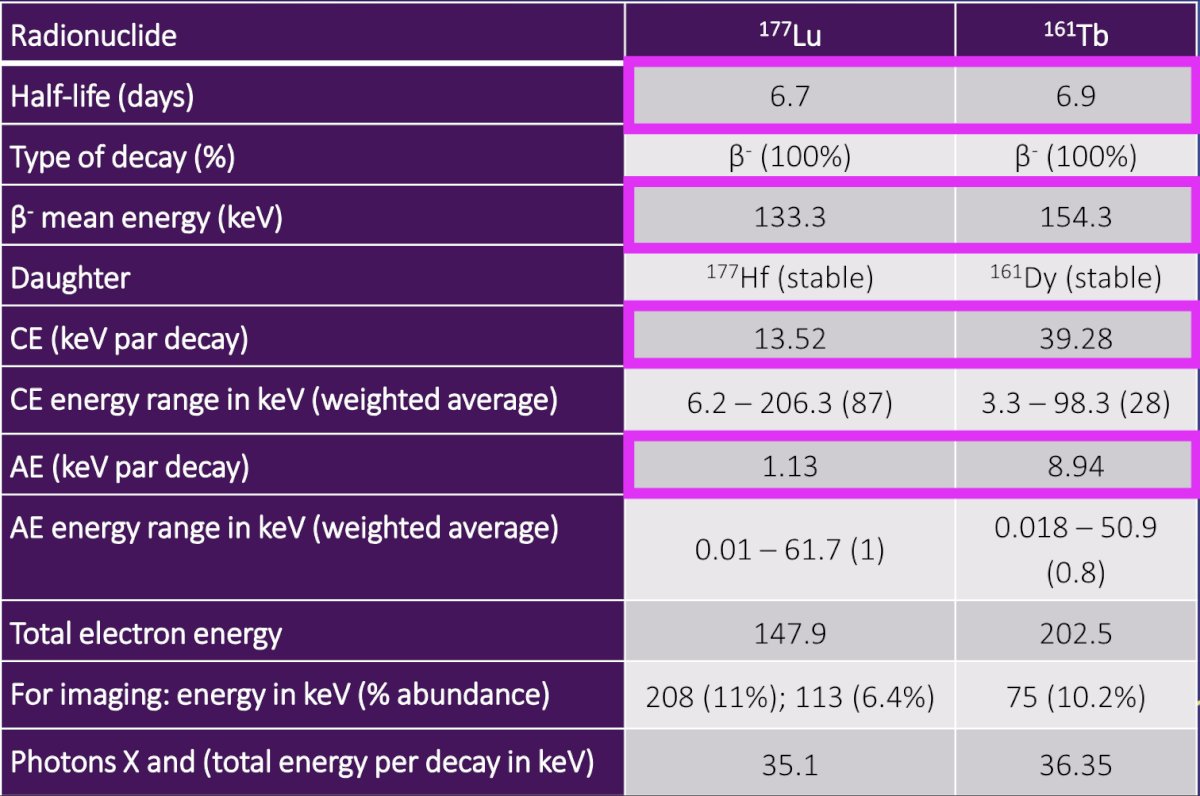
(UroToday.com) The 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) annual meeting featured a session on prostate cancer, and a presentation by Dr. James Buteau discussing radiation absorbed dose in patients with metastatic castration-resistant prostate cancer treated with 161Tb-PSMA-I&T. 177Lu-PSMA is a proven therapy for patients with metastatic castration-resistant prostate cancer. Terbium-161 attaches to PSMA receptors and emits beta-particles similar to 177Lu-PSMA, killing larger-sized tumors with abundant crossfire:
However, 161Tb also emits additional Auger electrons that delivers higher concentrations of radiation over very short path lengths, which may kill micrometastases better than 1177Lu-PSMA. Müller et al. [1] previously investigated 161Tb in combination with PSMA-617 as a potentially more effective therapeutic alternative to 177Lu-PSMA-617. 161Tb-PSMA-617 was tested in vitro and in tumor-bearing mice to confirm equal properties, as previously determined for 177Lu-PSMA-617. The relative tumor volume in mice over time for 161Tb-PSMA-617 versus 177Lu-PSMA-617 is as follows: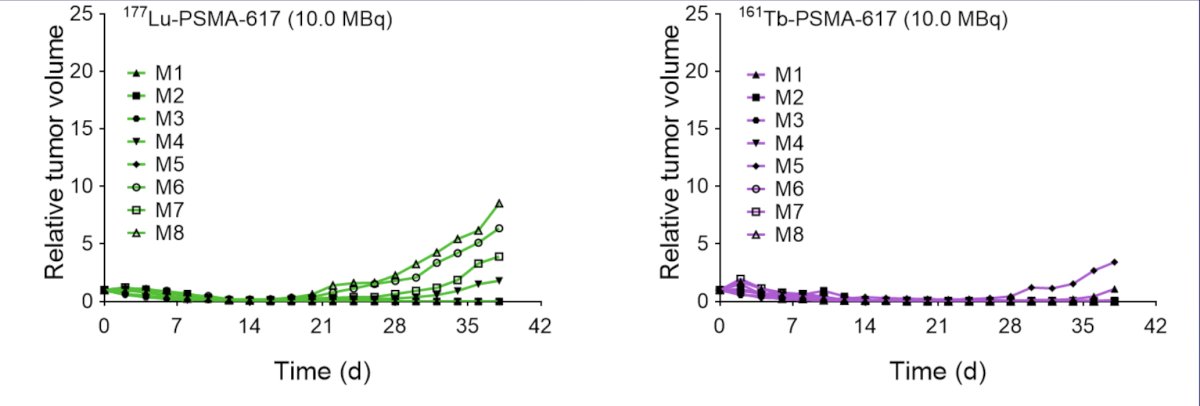
Post-therapy imaging and dosimetry are also feasible with low-energy gamma emission from 161Tb. The aim of this study was to calculate the radiation absorbed dose to normal organs in patients with metastatic castration-resistant prostate cancer who were treated with 161Tb-PSMA-I&T.
VIOLET (NCT05521412) is a single-center, single-arm, phase I/II trial recruiting 30 patients with progressive metastatic castration-resistant prostate cancer. The phase I dose-escalation is designed with a 3+3 model to establish the safest dose of 161Tb-PSMA-I&T (dose levels: 4.4, 5.5, and 7.4 GBq). In the dose escalation phase, a triple bed quantitative SPECT/CT from vertex to mid-thigh was acquired at three time-point intervals (2-6 hours, 18-24 hours, and 72-120 hours) after the first cycle of 161Tb-PSMA-I&T. Acquisitions were obtained on low-energy, high-resolution collimators, using a triple energy window peaked at 74 keV with upper and lower scatter limits. Retention of 161Tb-PSMA-I&T was estimated from voxel-based time-activity curves based on a multi-phase exponential clearance model and convolved using a GATE-derived voxel dose kernel based on decay of 161Tb in ICRP soft tissue to yield three-dimensional absorbed dose maps. Radiation absorbed dose to the respective organs was calculated for the beta particles using the voxel S values. The trial schema for VIOLET is as follows: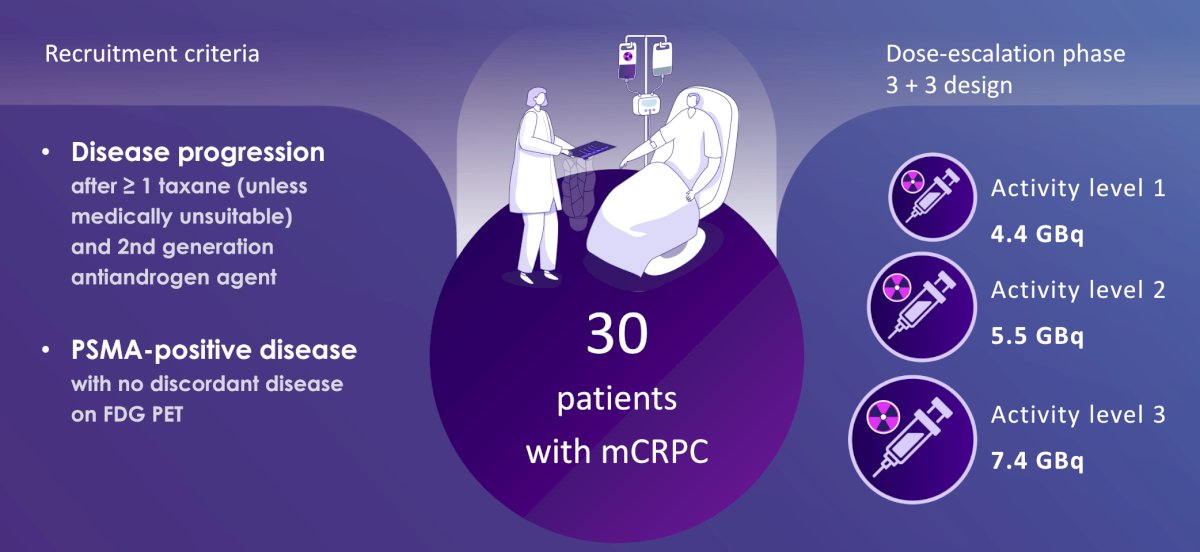
Overall, 12 patients received their first cycle of 161Tb-PSMA-I&T in the dose escalation phase. The quality of the SPECT/CT images was visually similar to those acquired post-177Lu-PSMA:
The mean total absorbed dose in the parotid glands was 0.92 (± 0.43), in the submandibular glands was 0.92 (± 0.41), in the kidneys was 2.21 (± 0.73), in the liver was 0.42 (± 0.15), and in the spleen was 0.38 (± 0.12) Gy. The mean absorbed dose per GBq for the same organs was 0.14 (± 0.05), 0.15 (± 0.06), 0.35 (± 0.10), 0.07 (± 0.03), and 0.06 (± 0.03) Gy/GBq, respectively. This was lower than recorded for 177Lu-PSMA: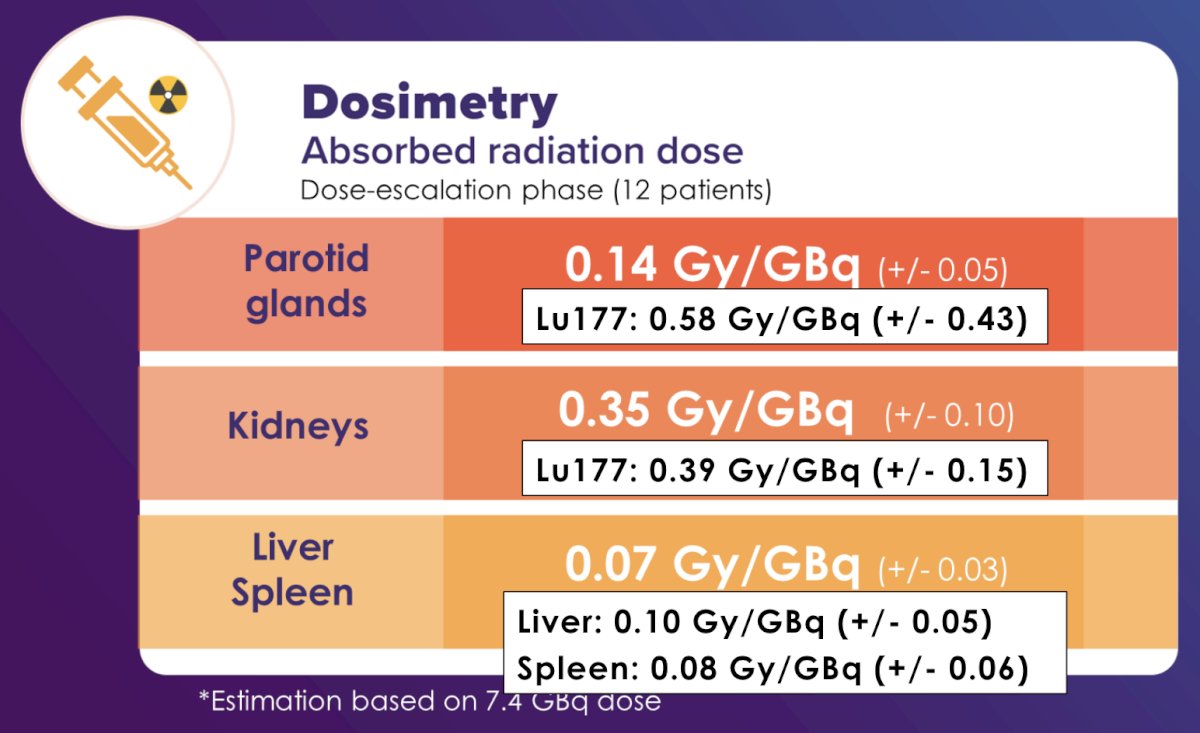
The response to 161Tb-PSMA-I&T in patient #1 (PSA decline to 5 cycles of treatment: 16 0.7 ng/mL):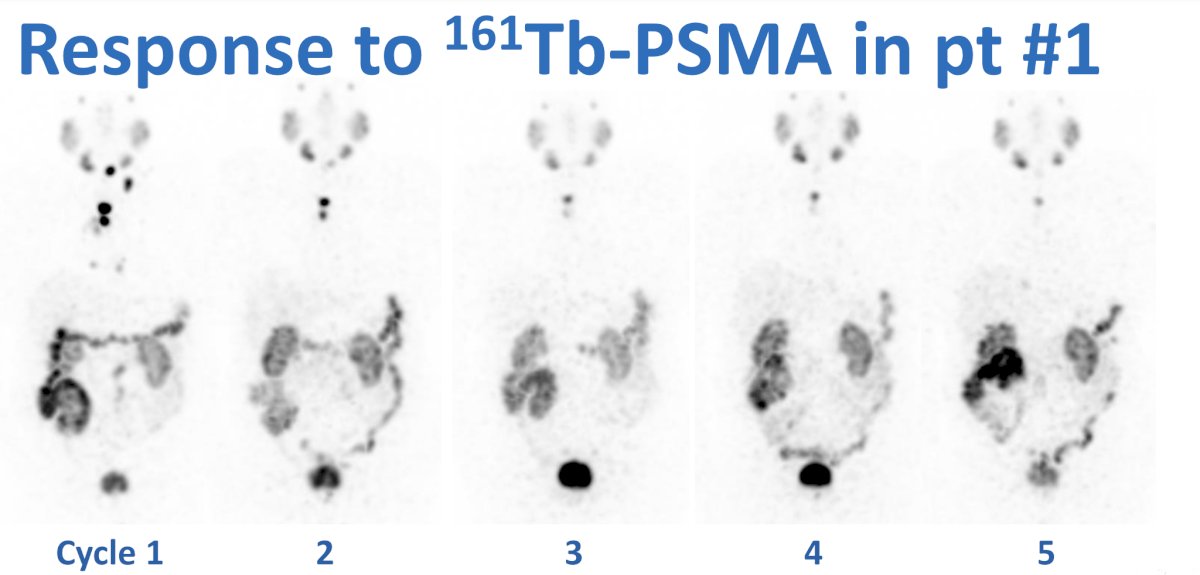
The strengths of the current study include (i) assessment of in-human biodistribution in a phase I/II trial, (ii) robust voxel-based dosimetry, and (iii) showing that QSPECT for 161Tb is feasible. Several weaknesses include the small sample size, as well as Auger and conversion electrons. Future directions will assess clinical outcomes and report on adverse events.
Dr. Buteau concluded his presentation discussing radiation absorbed dose in patients with metastatic castration-resistant prostate cancer treated with 161Tb-PSMA-I&T with the following take-home messages:
- Beta radiation absorbed dose following 161Tb-PSMA-I&T are within a safe and expected range for normal organs
- Additional radiation from Auger electrons is not accounted for in current models
Presented by: James P. Buteau, Research Fellow, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) Annual Meeting, Toronto, Ontario, Canada, Sat, June 8 – Tues, June 11, 2024.
Reference:
- Müller C, Umbricht CA, Gracheva N, et al. Terbium-161 for PSMA-targeted radionuclide therapy of prostate cancer. Eur J Nucl Med Mol Imaging. 2019 Aug;46(9):1919-1930.


