(UroToday.com) The 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) annual meeting featured a session on prostate cancer, and a presentation by Dr. Linlin Li discussing the dosimetry and pilot therapy study of novel PSMA-targeting agents, 177Lu-P17-087 and 177Lu-P17-088, in metastatic castration-resistant prostate cancer patients. PSMA is a promising target for diagnosis and radioligand therapy of prostate cancer. Biodistribution and dosimetry of two novel PSMA-targeting radionuclide therapy agents, 177Lu-P17-087, and its albumin binder-modified derivative, 177Lu-P17-088, were evaluated in metastatic castration-resistant prostate cancer patients:
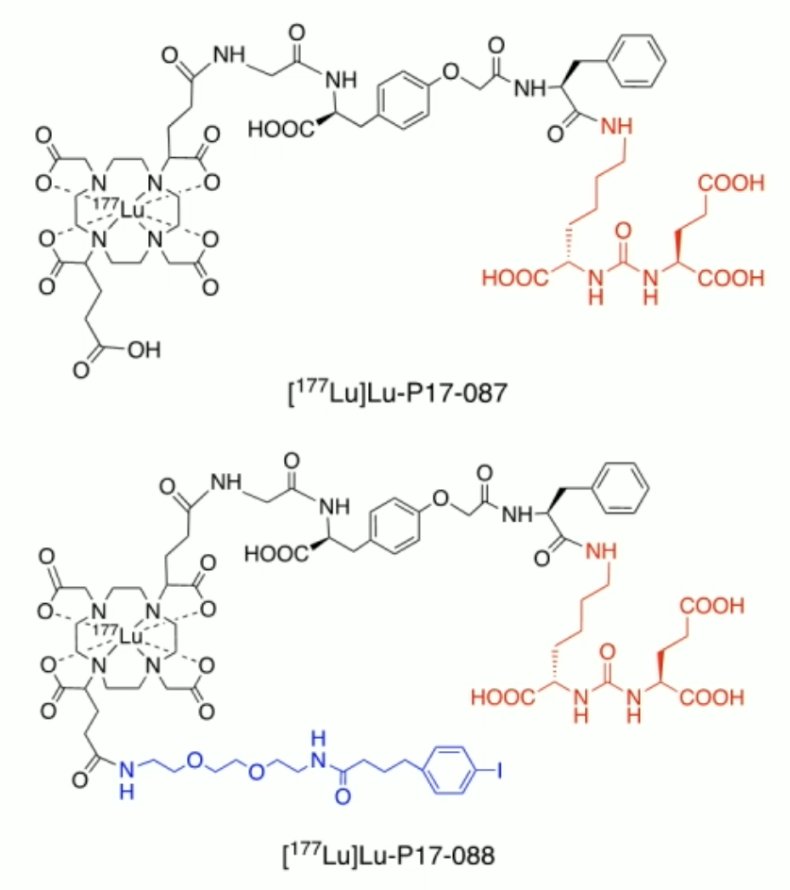
Additionally, the efficacy of single-dose 177Lu-P17-087 or 177Lu-P17-088 treatment in these patients was preliminarily evaluated.
Patients with PSMA-positive tumors were enrolled after a 68Ga-PSMA-11 PET/CT scan. Five metastatic castration-resistant prostate cancer patients received 177Lu-P17-087 and four other patients received 177Lu-P17-088 (1.20 GBq/patient). Each patient underwent SPECT scans at 1.5 hours, 4 hours, 24 hours, 48 hours, 72 hours, 120 hours, and 168 hours:
Biodistribution and radiation were calculated by Hermes software. For treatment study, two patients received 4.44 GBq/dose of 177Lu-P17-087, and two others received 2.59 GBq/dose of 177Lu-P17-088 for a cycle. General safety and therapeutic efficacy were assessed by follow-up interview, 68Ga-PSMA-11 PET/CT, and blood tests (including PSA). The study flow diagram is as follows: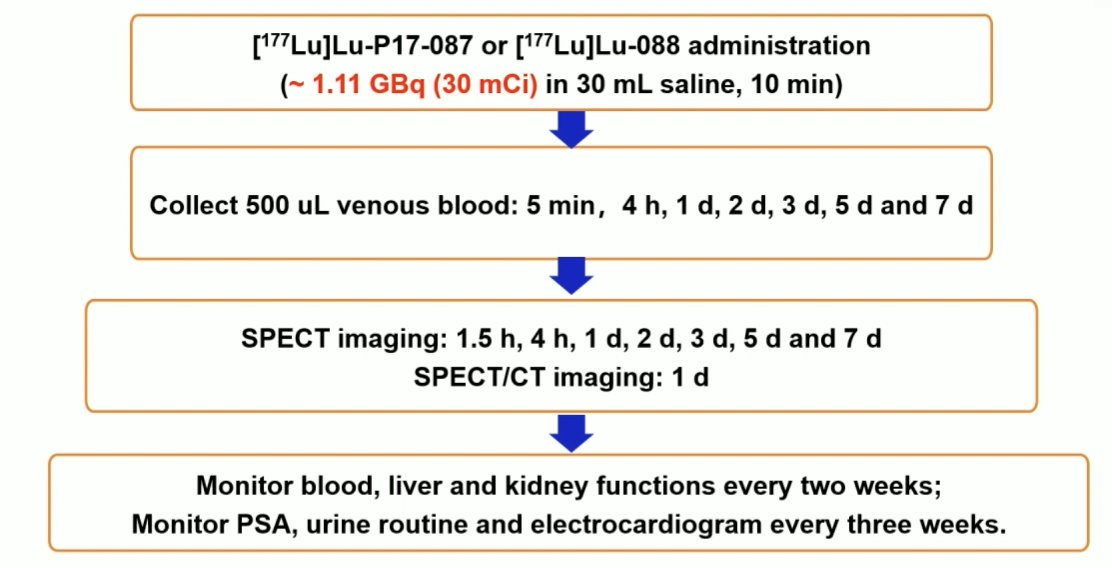
Patients showed no major clinical side effects under this study. As expected 177Lu-P17-088 with longer blood circulation (due to its albumin binding) exhibited higher effective doses than 177Lu-P17-087 (0.151 ± 0.036 versus 0.056 ± 0.019 mGy/MBq, p = 0.001). Similarly, red marrow received 0.119 ± 0.068 and 0.048 ± 0.020 mGy/MBq, while kidney doses were 0.119 ± 0.068 and 0.046 ± 0.022 mGy/MBq, respectively: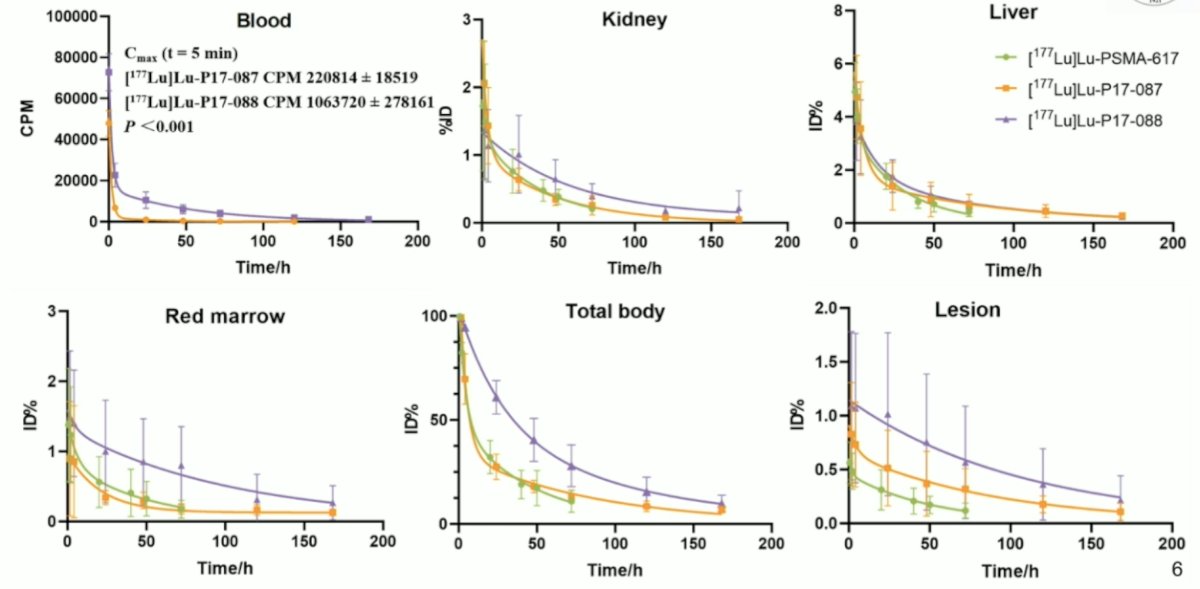
177Lu-P17-087 demonstrated excellent tumor uptake and faster kinetics, while 177Lu-P17-088 displayed a slower washout and higher average dose (7.75 ± 4.18 versus. 4.72 ± 2.29 mGy/MBq, p = 0.018). After administration of 1.2 GBq 177Lu-P17-087 or 177Lu-P17-088, 3/5 and 3/4 patients showed reducing PSA values, respectively. For one cycle treatment study, four patients displayed decreased PSA response and two of them (treated with 177Lu-P17-087 and 177Lu-P17-088, respectively) declined by more than 50%. 68Ga-PSMA-11 PET/CT showed significant response in both treatment groups, the change of total lesion PSMA were -37.65% for 177Lu-P17-087 group and -27.25% for 177Lu-P17-087 group.
Dr. Li concluded this presentation discussing the dosimetry and pilot therapy study of 177Lu-P17-087 and 177Lu-P17-088 in metastatic castration-resistant prostate cancer patients with the following take-home messages:
- 177Lu-P17-087 and 177Lu-P17-088 are two promising radioligand therapy agents
- 177Lu-P17-088 may have an enhanced PSMA-targeting dose delivery and desirable therapeutic index
- 177Lu-P17-087 and 177Lu-P17-088 may have better PSMA targeting dose delivery than 177Lu-PSMA-617
- Low dose 177Lu-P17-087 and 177Lu-P17-088 (1.2 GBq/patient) appeared to show encouraging PSA response rates
Presented by: Linlin Li, Researcher, Peking Union Medical College Hospital, Beijing, China
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) Annual Meeting, Toronto, Ontario, Canada, Sat, June 8 – Tues, June 11, 2024.


