(UroToday.com) The 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) annual meeting featured a session on prostate cancer, and a presentation by Dr. Jean-Mathieu Beauregard discussing a sub-analysis of the 3TMPO study assessing eligibility for PSMA radioligand therapy based on dual FDG/PSMA-PET. Eligibility criteria for PSMA radioligand therapy are currently debated, particularly with respect to the role of FDG-PET in patient selection.
Dr. Beauregard and colleagues are proposing new imaging eligibility criteria for PSMA radioligand therapy based on dual-tracer FDG/PSMA-PET that do not rely on lesion size on CT. The aims of this analysis were to compare these new criteria with those of the TheraP1 and VISION2 trials when applied in a prospective cohort of patients with metastatic castration-resistant prostate cancer and to correlate the radioligand therapy eligibility with overall survival.
There were 98 patients with progressive metastatic castration-resistant prostate cancer and at least three metastases on conventional imaging who were enrolled in 3TMPO, a multicenter prospective cohort study investigating the intrapatient intermetastatic heterogeneity with multi-tracer PET/CT (NCT04000776). All participants underwent 18F-FDG and 68Ga-PSMA-617 PET/CT within ten days. Lesions that were analyzed had a molecular tumor volume ≥1 cc on either PET when segmented using a threshold of 1.5x liver SUVmean. For each lesion and tracer, the ratio of SUVpeak to liver SUVmean (SUVR) was computed.
Eligibility to radioligand therapy was defined as having at least one PSMA+ lesion and no FDG+/PSMA– lesion. The investigators assessed different positivity thresholds defining radioligand therapy eligibility: (i) the protocol’s prespecified threshold of SUVR ≥1.5 for both tracers (3TMPOA), and (ii) post-hoc, relaxed thresholds of SUVR ≥2.0 for FDG and of SUVR ≥1.0 for PSMA (3TMPOB). Radioligand therapy eligibility according to TheraP criteria (i.e. ≥1 lesion with SUVmax ≥20, all CT-measurable lesions/nodes ≥10 mm with SUVmax ≥10, no FDG+ lesion with PSMA SUVmax <10 or FDG>PSMA) and VISION (i.e. ≥1 lesion with PSMA uptake > liver; no bone super scan, no CT-measurable soft-tissue lesion ≥10 mm or node ≥25 mm with PSMA uptake < liver) was determined independently by two expert readers who reached a consensus on discordant cases. Overall survival was derived from Kaplan-Meier curves and log-rank test was used for comparisons.
Application of the 3TMPOA radioligand therapy eligibility criteria, i.e. excluding patients harboring FDG+(SUVR≥1.5)/PSMA–(SUVR<1.5) lesions, resulted in 52 (53%) participants being deemed eligible to PSMA radioligand therapy. Application of the relaxed 3TMPOB radioligand therapy eligibility criteria, i.e. excluding patients harboring FDG+ (SUVR ≥ 2.0)/PSMA– (SUVR < 1.0) lesions, increased this number to 69 (70%). In comparison, 39 (40%) and 75 (77%) participants would have been deemed eligible to radioligand therapy based on TheraP and VISION criteria, respectively. After a median follow-up of 12.3 months (95% CI 12.1–12.4 months), the median overall survival of the cohort was 10.2 months (95% CI 8.5–11.8 months.). For all criteria sets, the median overall survival of radioligand therapy-eligible participants was superior to that of ineligible ones, with significant to near-significant reductions in risk of death (HR 0.38–0.63, p = 0.0002–0.10):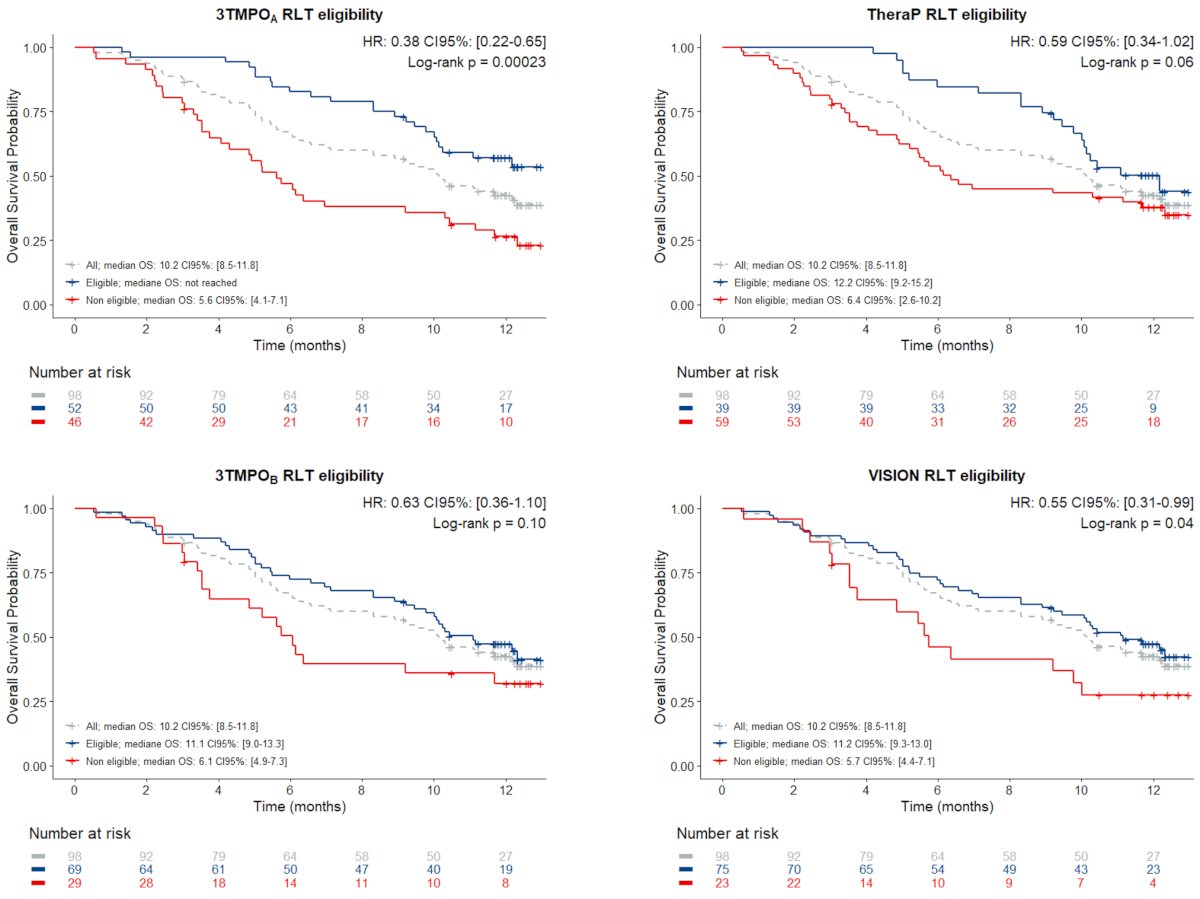
Of note, the median overall survival of the fifteen 3TMPOA-eligible/TheraP-ineligible participants was not reached, while that of the two 3TMPOA-inelegible/TheraP-eligible participants was 5.2 months. Similarly, the median overall survival of the four 3TMPOB-eligible/VISION-ineligible participants was 9.8 months, while that of the ten 3TMPOB-ineligible/VISION-eligible participants was 6.1 months.
The first case example is that of a 64-year-old with metastatic castration-resistant prostate cancer who was ineligible for radioligand therapy based on TheraP criteria (no lesion with PSMA SUVmax >= 20), but eligible according to 3TMPOA, 3TMPOB, and VISION. He received radioligand therapy and was still alive at the 13-month follow-up:
The second case example is that of a 67-year-old with metastatic castration-resistant prostate cancer who was eligible for radioligand therapy based on VISION criteria, but ineligible based on 3TMPOA, 3TMPOB, and TheraP (FDG+/PSMA- bone lesions). He received radioligand therapy but deceased at 5.2 months: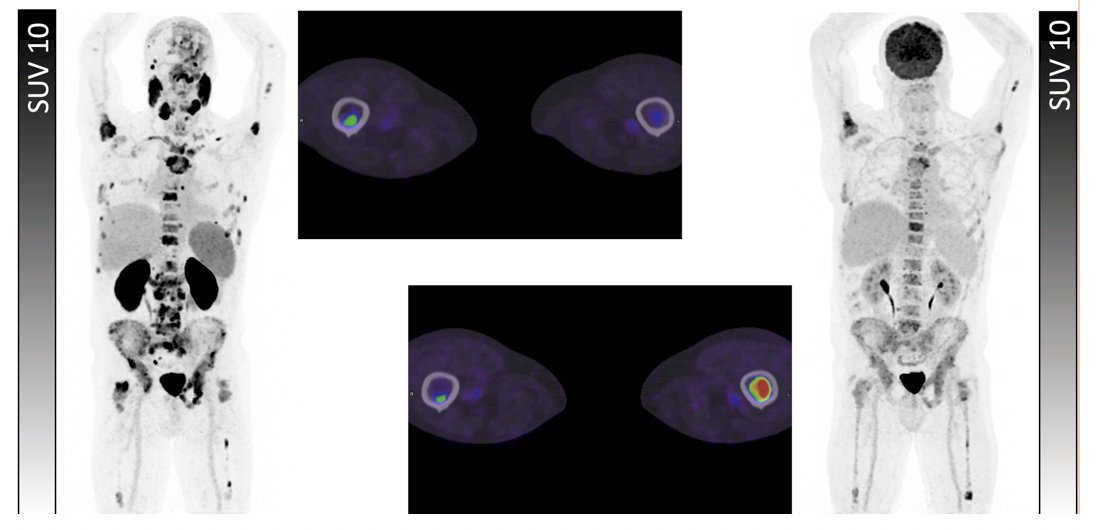
Dr. Beauregard concluded his presentation discussing a sub-analysis of the 3TMPO study assessing eligibility to PSMA radioligand therapy based on dual FDG/PSMA-PET with the following take-home messages:
- The study proposes two novel dual FDG/PSMA PET-based radioligand therapy eligibility criteria sets – one more selective and one more permissive – that would both allow more metastatic castration-resistant prostate cancer patients to receive PSMA radioligand therapy than the TheraP criteria, yet would still exclude those patients with poorly targeted, significantly hypermetabolic lesions (including bone metastases) otherwise permitted by the VISION criteria and associated with a poor prognosis
- This pragmatic, dual-PET quantitative criteria sets thus appear reasonably balanced between the most frequently cited ones, and their adoption in clinical trials would allow to prospectively fill the knowledge gap regarding the benefits of PSMA radioligand therapy in patients harboring FDG+ lesions with borderline PSMA expression
Presented by: Jean-Mathieu Beauregard, MD, MSc, FRCPC, CHU de Quebec, Université Laval, Quebec City, Quebec, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) Annual Meeting, Toronto, Ontario, Canada, Sat, June 8 – Tues, June 11, 2024.
References:
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.


