(UroToday.com) The 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) annual meeting featured a session on prostate cancer, and a presentation by Dr. Raghava Karri discussing the prognostic value of post-therapy SPECT/CT imaging in patients undergoing 177Lu-PSMA-617 (Lu-PSMA) radioligand therapy. Despite established efficacy of Lu-PSMA, as evidenced by the TheraP1 and VISION2 trials, there remains a notable gap in understanding the utility of post-therapy imaging. At the 2024 SNMMI annual meeting, Dr. Karri and colleagues reviewed changes on post-therapy SPECT/CT as a prognostic biomarker compared to PSA and conventional prognostic parameters in three clinical trials.
This was a retrospective analysis of post-therapy SPECT/CT images of patients receiving at least two cycles of Lu-PSMA therapy from three clinical trials. Patients from a Peter MacCallum phase 2 (n = 46), PRINCE (n = 25), and LuPARP (n = 14) studies were included. Molecular tumor volume was contoured on the post-therapy images with an SUV cut-off of 3. Total lesion activity was derived as a product of molecular tumor volume and SUVmean, and PET parameters were correlated with PSA change at cycle 2 (Pearson’s correlation coefficient). Prognostic value of new lesions (detected visually) on SPECT/CT at cycle 2, change in molecular tumor volume, total lesion activity, and SUVmax at cycle 2 on overall survival was assessed using Cox proportional hazard model, adjusting by age, Gleason score and change in PSA.
There were 85 patients with metastatic castration-resistant prostate cancer analyzed with a median age of 71 (IQR 67-75) years. There was moderate correlation between change in PSA from cycle 1 to cycle 2 and molecular tumor volume (r = 0.56 [95% CI 0.40 - 0.69] p < 0.001) or total lesion activity (r = 0.55 [0.39 - 0.69] p < 0.001 for total lesion activity). Reduction in total lesion activity (HR 0.98, 95% CI 0.97, 1.00; p = 0.016) and molecular tumor volume (HR 0.98, 95% CI 0.96, 1.00; p = 0.009; per 10% increased for both) were associated with overall survival on univariable analysis but not in multivariate analysis when adjusting for clinical factors. Changes in SUVmax and SUVmean were not associated with overall survival (HR 0.98, 95% CI 0.93 - 1.03; p = 0.38 and HR 0.98, 95% CI 0.86 - 1.11; p = 0.71, respectively). There were 18 of 85 (21.2%) patients that had new lesions on cycle 2 SPECT/CT, and this was prognostic for overall survival in univariate and multivariate analysis (HR 2.38, 95% CI 1.36 - 4.18, p = 0.002; HR 2.85, 95% CI: 1.36 - 5.98, p = 0.01):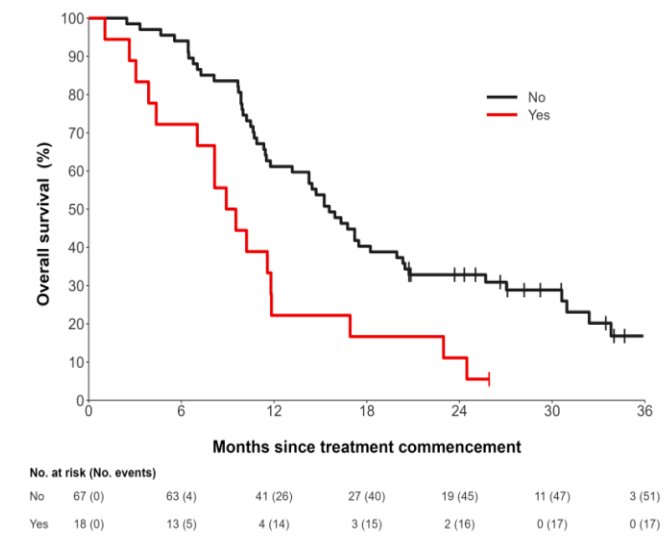
Notably, 7/85 (8.2%) of patients with PSA reduction had new lesions developing on the post-therapy SPECT/CT images.
Dr. Karri concluded his presentation discussing the prognostic value of post-therapy SPECT/CT imaging in patients undergoing LuPSMA radioligand therapy with the following take-home messages:
- Volumetric parameters on SPECT/CT (molecular tumor volume, total lesion activity) showed prognostic value for overall survival in univariate analysis
- New lesions on SPECT/CT were strongly prognostic in uni- and multivariate analysis
- This suggests that SPECT/CT findings are clinically relevant as they may be visible even in patients with declining PSA
Presented by: Raghava K. Karri, MD, Nuclear Medicine Physician, Peter MacCallum Cancer Centre, Melbourne, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WWellStarMCG Health, @zklaassen_md on Twitter during the 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) Annual Meeting, Toronto, Ontario, Canada, Sat, June 8 – Tues, June 11, 2024
References:
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.


