(UroToday.com) The 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) annual meeting featured a session on prostate cancer, and the Barry Siegel Award Lecture presentation by Dr. Steven Rowe discussing registrational trials for PSMA PET. Dr. Rowe started his presentation highlighting that PSMA is a transmembrane carboxypeptidase highly expressed on prostate cancer cells with frequent expression observed in prostate cancer tumors. Furthermore, there has been direct correlation between expression levels and tumor aggressive. What we know so far about PSMA PET is that it has (i) moderate sensitivity and very high specificity for preoperative nodal staging, (ii) high detection efficiency for sites of biochemical recurrence, and (iii) effective for guiding focal therapy and selecting patients for PSMA-based therapeutic molecules. 18F-DCFPyL in high risk prostate cancer patients can have vastly different staging results:

Dr. Rowe then discussed the key registrational trials for PSMA staging of high risk patients. In 2021, results of the Observe Prostate Cancer with Accuracy (OSPREY) trial were published.1 This was a prospective, multicenter phase 2/3 trial that included two distinct cohorts (Cohorts A and B), of which Cohort A included 268 men with newly diagnosed high-risk prostate cancer (i.e., clinical stage ≥T3a or PSA >20 ng/ml or Gleason score ≥8) planned for radical prostatectomy with lymph node dissection. The sensitivity and specificity of 18F-DCFPyL-PET/CT were 40% and 98%, respectively. The positive and negative predictive values were 87% and 83%, respectively. Compared to conventional CT, 18F-DCFPyL-PET/CT demonstrated higher specificity (98% versus 65%), more than threefold higher positive predictive value (87% versus 28%), higher specificity (98% versus 65%), and similar sensitivity (40% versus 43%). Detection outcomes in the OSPREY trial are as follows:
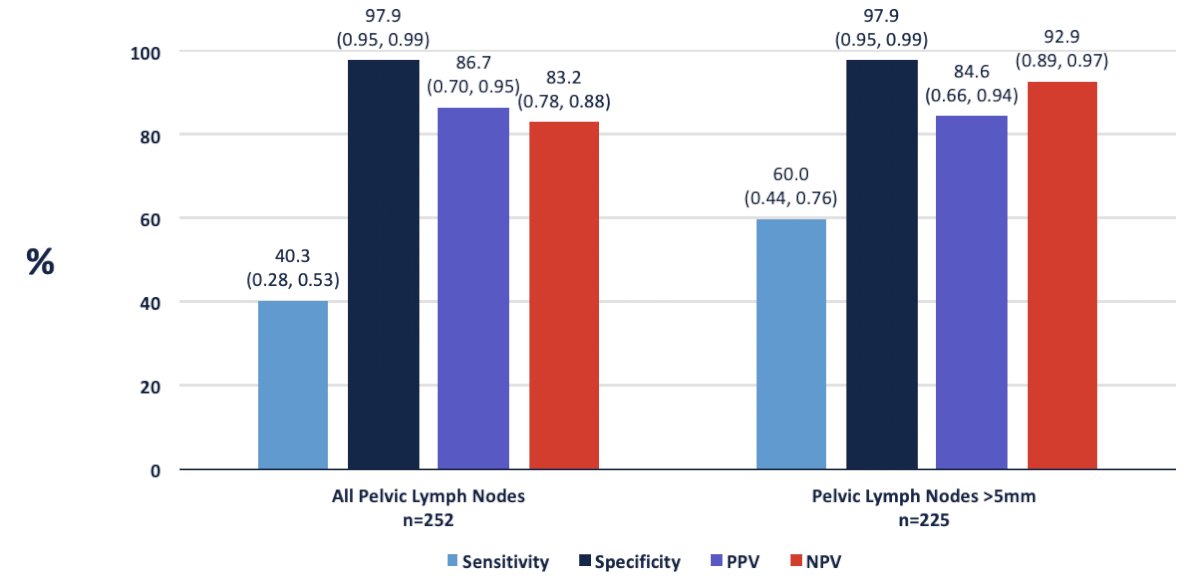
Hope et al.2 published in 2021 a phase 3 imaging trial assessing the accuracy of 68Ga-PSMA-11 PET imaging for the detection of pelvic nodal metastases compared with histopathology at time of radical prostatectomy and pelvic lymph node dissection. In this trial, there were 764 men that underwent a 68Ga-PSMA-11 PET imaging scan for primary staging, and 277 of 764 (36%) subsequently underwent prostatectomy with lymph node dissection. Based on pathology reports, 75 of 277 patients (27%) had pelvic nodal metastasis. Results of 68Ga-PSMA-11 PET/CT were positive in 40 of 277 (14%), 2 of 277 (1%), and 7 of 277 (3%) of patients for pelvic nodal, extrapelvic nodal, and bone metastatic disease, respectively. Sensitivity, specificity, positive predictive value, and negative predictive value for pelvic nodal metastases were 0.40 (95% CI, 0.34-0.46), 0.95 (95% CI, 0.92-0.97), 0.75 (95% CI, 0.70-0.80), and 0.81 (95% CI, 0.76-0.85), respectively.
In the LIGHTHOUSE trial, the objective was to evaluate the diagnostic performance and safety of 18F-rhPSMA-7.3 in newly diagnosed prostate cancer patients planned for prostatectomy.3 Among 352 patients, the sensitivity for pelvic lymph node dissection detection was 30% (95% CI, 19.6-42.1%) for reader 1, 27% (95% CI, 17.2-39.1%) for reader 2, and 23% (95% CI, 13.7-34.4%) for reader 3, not meeting the prespecified threshold. Specificity was 93% (95% CI, 88.8-95.9%), 94% (95% CI, 89.8-96.6%), and 97% (95% CI, 93.7-98.7%), respectively, exceeding the threshold for all readers. Specificity was high (≥ 92%) across both risk stratifications.
Moving to the biochemical recurrent disease space and the utility of PSMA PET/CT, Dr. Rowe discussed the CONDOR trial,4 which was a prospective phase III trial that evaluated 18F-DCFPyL-PET/CT in men with biochemical recurrence following prostatectomy or radiotherapy, defined as PSA ≥ 0.2 ng/ml after radical prostatectomy and PSA ≥ 2 ng/ml post-radiation. All patients in CONDOR had negative or equivocal conventional imaging (including 18F-fluciclovine or 11C-choline PET, CT, MRI, and/or whole-body bone scintigraphy) within 60 days prior to 18F-DCFPyL-PET/CT. This trial included 208 patients with a median PSA level of 0.8 ng/ml (range: 0.2 – 98.4). 18F-DCFPyL-PET/CT was positive in 59.1-65.9% of patients as assessed by the three independent blinded central readers. The correct localization rate ranged between 84.8% and 87.0% among the three readers. There was a clear association between increasing baseline PSA level and both improved detection and correct localization rates:
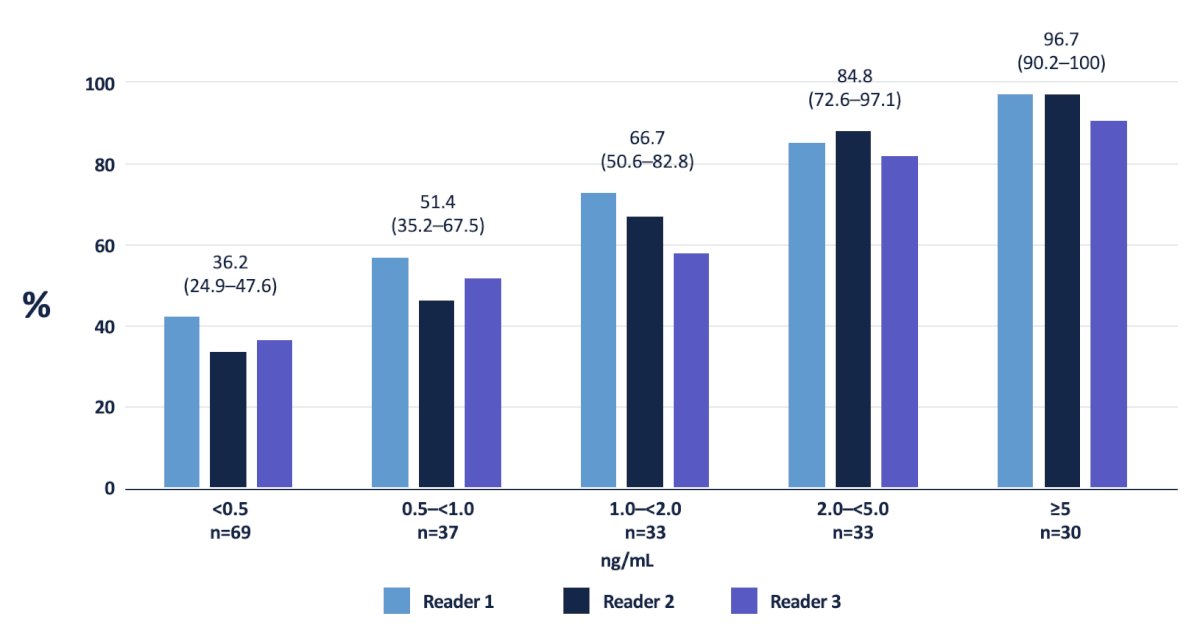
The correct localization rates stratified by PSA in the CONDOR trial is as follows:

In 2019, Fendler and colleagues5 assessed 68Ga-PSMA-11 PET/CT accuracy in a prospective multicenter trial among 635 patients with biochemically recurrent prostate cancer after prostatectomy (n = 262, 41%), radiation therapy (n = 169, 27%), or both (n = 204, 32%). On a per-patient basis, positive predictive value was 0.84 (95% CI, 0.75-0.90) by histopathologic validation and 0.92 (95% CI, 0.88-0.95) by the composite reference standard (n = 217), with 68Ga-PSMA-11 PET/CT localizing recurrent prostate cancer in 475 of 635 (75%) patients.
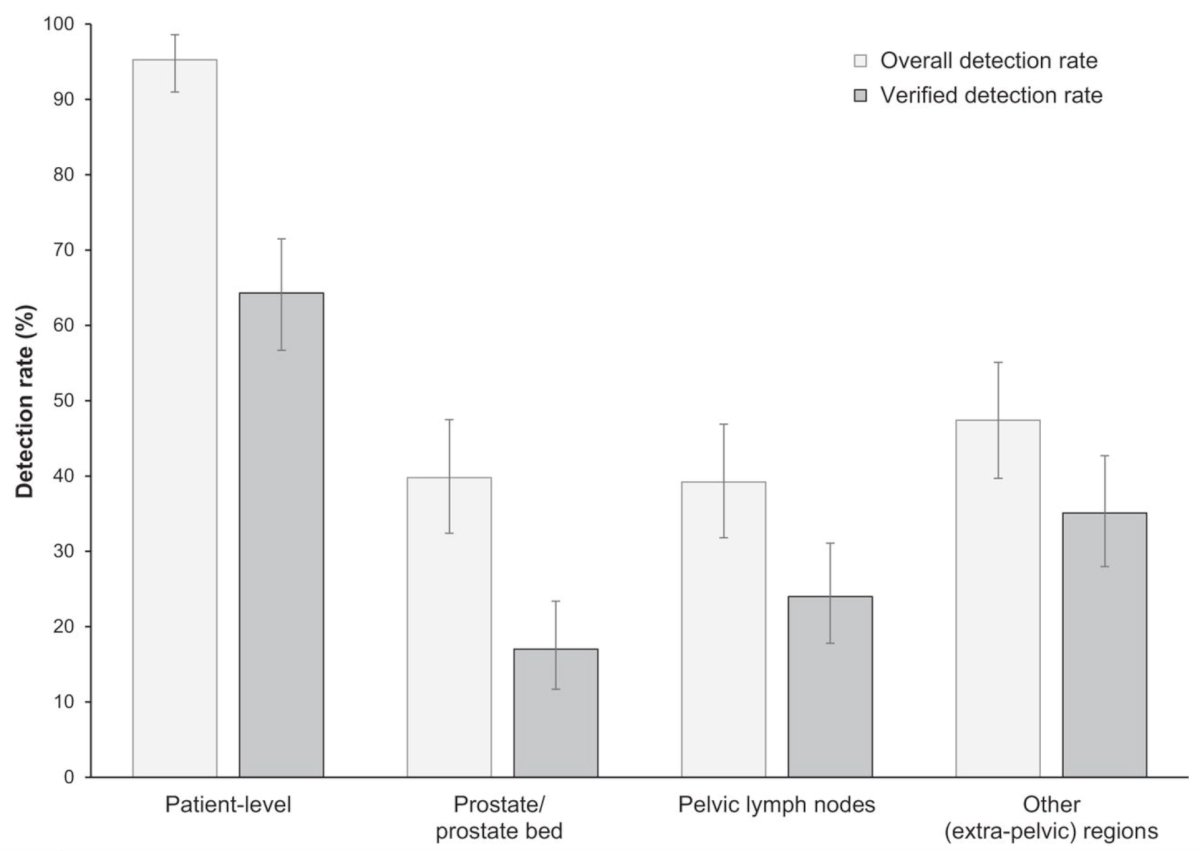
In a recent subsequent analysis of the SPOTLIGHT trial,6 Fleming et al. assessed the performance of 8F-flotufolastat PET/CT for identifying PSMA-positive lesions confirmed by standard of truth in men with biochemical recurrence of prostate cancer and negative conventional imaging at baseline. Among 171 patients with negative baseline conventional imaging and standard of truth by histopathology or post-PET confirmatory imaging, the overall 18F-flotufolastat detection rate among these patients was 95% (95% CI, 91.0%-98.0%), and 110 of 171 of these patients had at least 1 true-positive lesion identified (verified detection rate, 64%; 95% CI, 56.7%-71.5%):
Dr. Rowe then discussed OSPREY Cohort B,1 which included 117 patients with presumptive radiological evidence of recurrent or metastatic prostate cancer on conventional imaging and in whom lesions were considered amenable to biopsy. At the time of study entry, conventional imaging demonstrated locoregional disease in 28% of patients and distant disease in the remaining 72%. The sensitivity and positive predictive value of 18F-DCFPyL-PET/CT in this setting were 96% and 82%, respectively. Significantly, this test performed similarly regardless of site of metastasis:

Dr. Rowe then highlighted several limitations of 18F-DCFPyL-PSMA PET/CT in metastatic prostate cancer, including:
1. Neuroendocrine differentiation:
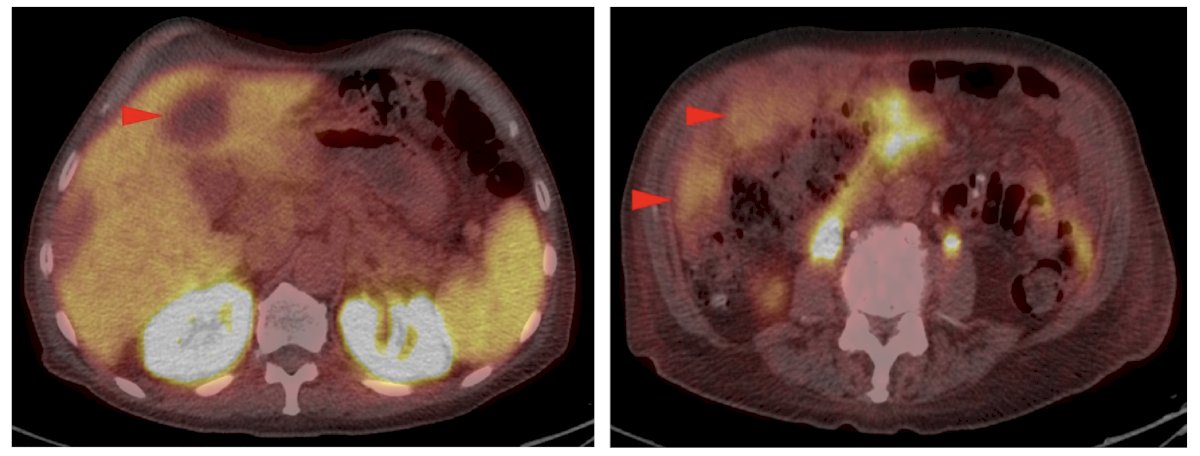
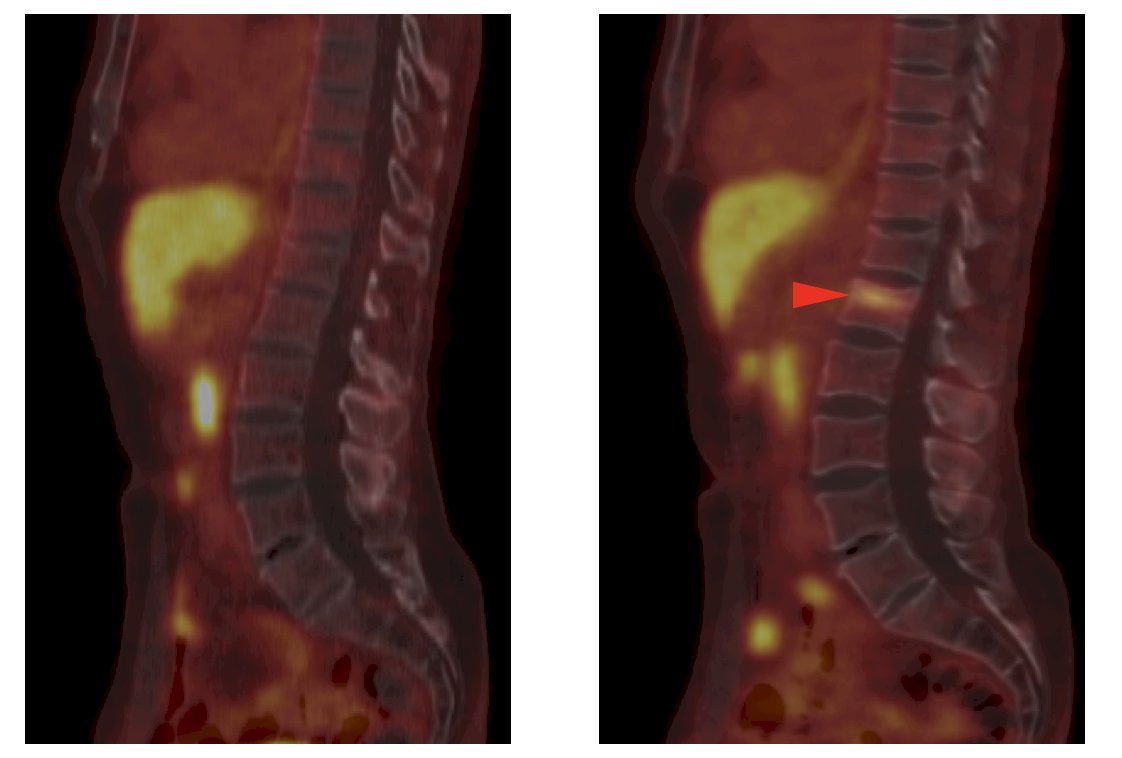
2. Uptake at the site of a non-pathologic fracture:
3. Uptake in peripheral ganglia:
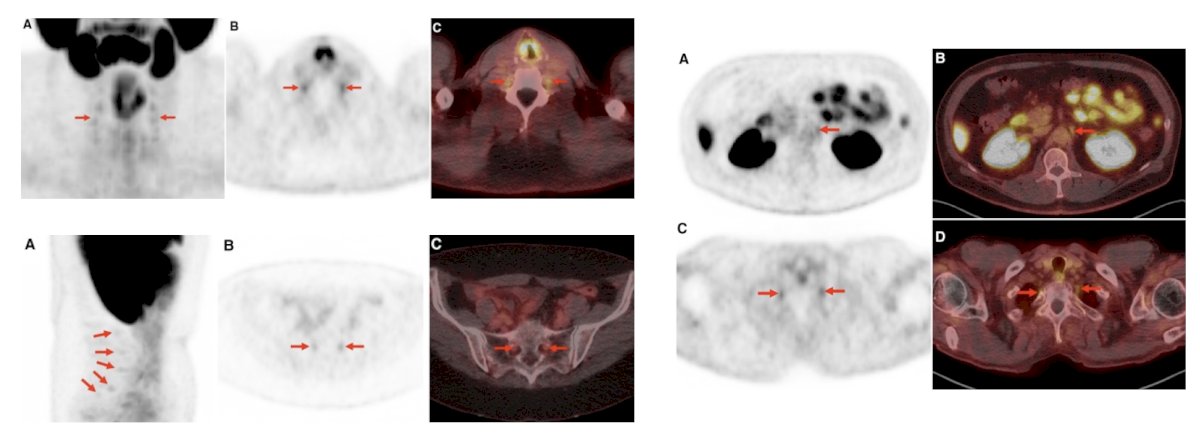
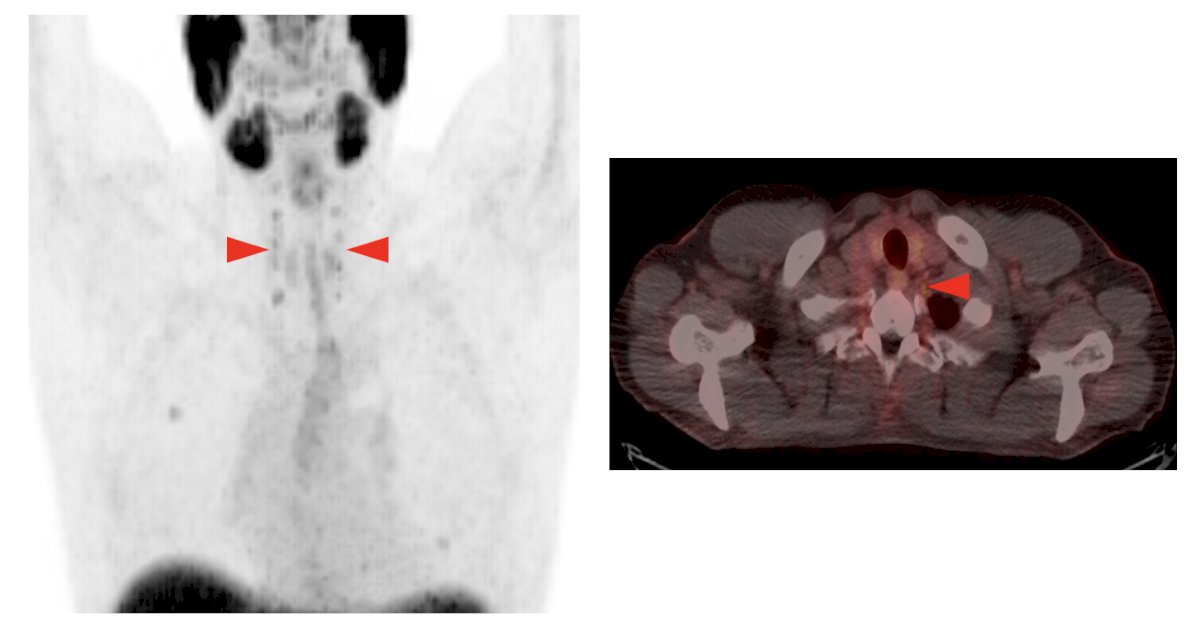
4. Uptake in ganglion structures (dorsal root and stellate ganglia):
5. Uptake in ganglion structures (celiac ganglia):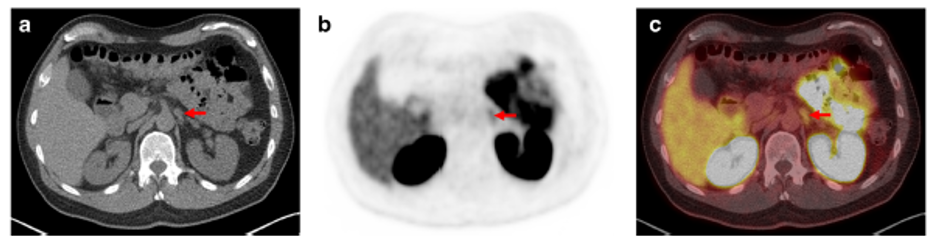
6. Uptake in Paget’s disease of the bone:
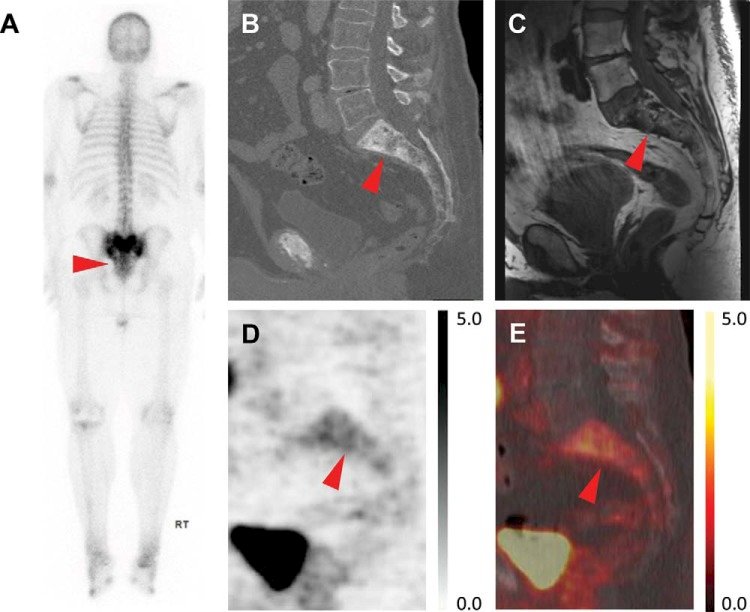
7. Uptake in post-radiation changes (cortical laminar necrosis):

Dr. Rowe then discussed theranostics, specifically 177Lu-PSMA based radioligand therapy. A 2021 meta-analysis of trials assessing 177Lu-PSMA based radioligand therapy reported several important findings:7
- Proportion with >= 50% PSA decrease after treatment with PSMA I&T, 617, EB-617, iPSMA: 42% (95% CI 37-48%)
- Proportion with any PSA decrease after treatment with PSMA I&T, 617, EB-617, iPSMA: 70% (95% CI 65-74%)
- Proportion with neuropathy, any grade, after treatment with PSMA I&T, 617, EB-617, iPSMA: 12% (95% CI 6-22%)
- Proportion with xerostomia, any grade, after treatment with PSMA I&T, 617, EB-617, iPSMA: 21% (95% CI 12-35%)
These smaller studies were the lead in/lead up for the phase 3 registration trial, the VISION trial.8 VISION was an international, randomized, open-label phase III study evaluating 177Lu-PSMA-617 in men with PSMA-positive mCRPC who had previously received treatment with a next-generation androgen receptor signaling inhibition (abiraterone, enzalutamide, etc) and one or two prior lines of taxane chemotherapy. Patients must have had an ECOG performance status of 0-2 and life expectancy of at least 6 months. Importantly, patients must have had PSMA-positive disease on the basis of a central review of 68Ga-PSMA-11 staging scans. PSMA positivity was defined as uptake greater in metastatic lesions than in the liver. Further, they could have no PSMA-negative metastatic lesions. Following enrollment, patients were randomized in a 2:1 fashion to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks x 6 cycles) plus standard of care or standard of care alone. Standard of care treatments was at the discretion of the treating investigator. However, cytotoxic chemotherapy, immunotherapy, and radium-223 were explicitly excluded. Most patients received alternative androgen-directed therapies while others received palliative radiotherapy and glucocorticoids.
This trial assessed two alternate primary endpoints: (i) rPFS using the Prostate Cancer Working Group 3 (PCWG3) criteria by independent central review and (ii) OS. Secondary endpoints included ORR (RECIST v1.1), disease control rate, time to first symptomatic skeletal event, and safety/AE profile. VISION enrolled 831 patients between June 2018 and October 2019. In keeping with the 2:1 randomization schema, 551 patients were allocated to 177Lu-PSMA-617 + standard of care, and 280 were allocated to standard of care only. Over a median study follow-up of 20.9 months, treatment with 177Lu-PSMA-617 + standard of care significantly improved overall survival by a median of 4.0 months (median overall survival: 15.3 vs 11.3 months; HR 0.62, 95% CI 0.52 to 0.74; p < 0.001, one-sided), compared to standard of care alone, in the overall cohort of all randomized patients (n = 831): 
With regards to the other primary endpoint of radiographic progression free survival, treatment with 177Lu-PSMA-617 + standard of care significantly improved radiographic progression free survival by a median 5.3 months (median, 8.7 vs 3.4 months; HR 0.40, 99.2% CI 0.29 to 0.57; p < 0.001, one-sided):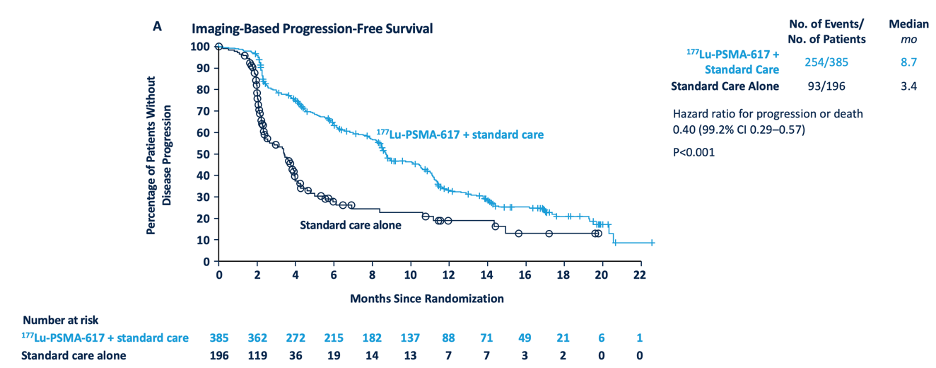
In addition to these primary endpoints, the addition of 177Lu-PSMA-617 to standard of care statistically significantly improved all key secondary endpoints, including objective response rate (29.8% vs 1.7%), disease control rate (89.0% vs 66.7%) and time to first symptomatic skeletal event (median time: 11.5 vs 6.8 months; HR 0.50, 95% CI 0.40 to 0.62):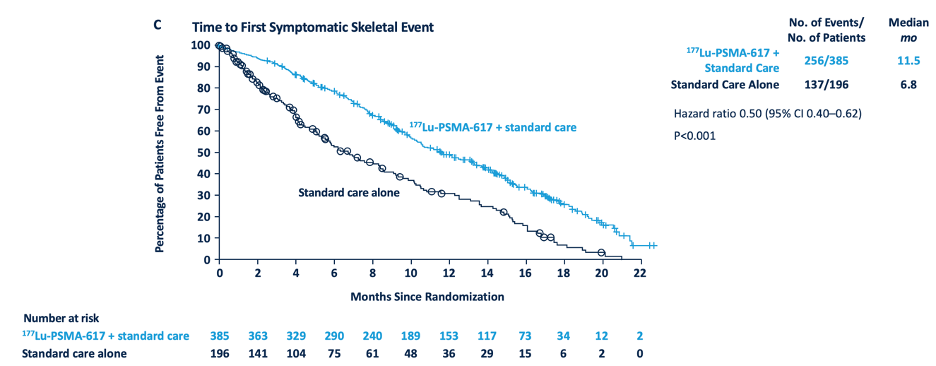
Dr. Rowe then discussed emerging ideas and what we need from future trials:
- It is not all about sensitivity and specificity
- We need predictive biomarkers derived from imaging and clinical data
- We need to understand response assessment and incorporate serial PSMA PET into therapeutic clinical trials
- We need to incorporate artificial intelligence
How PSMA PET/CT’s look after prior therapy is still an area of investigation. Dr. Rowe was the senior author of a prospective, single-arm trial evaluating changes in uptake patterns on PSMA-targeted 18F-DCFPyL PET/CT in patients with castration resistant prostate cancer starting abiraterone or enzalutamide.9 Patients were imaged with 18F-DCFPyL PET/CT immediately prior to starting abiraterone or enzalutamide and 2-4 months later: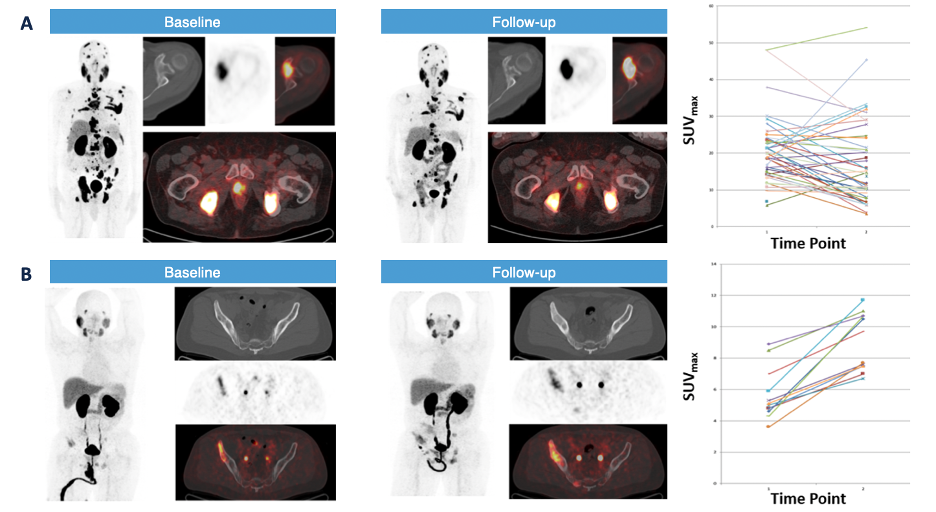
Among 16 evaluable patients, the median time to therapy change was 9.6 months (95% CI, 6.9-14.2), and median overall survival was 28.6 months (95% CI, 18.3- NA). Patients with a mixed but predominantly increased pattern of radiotracer uptake had a shorter time to therapy change and overall survival. Moreover, the percentage change in PSA stratified by response pattern on PET is as follows: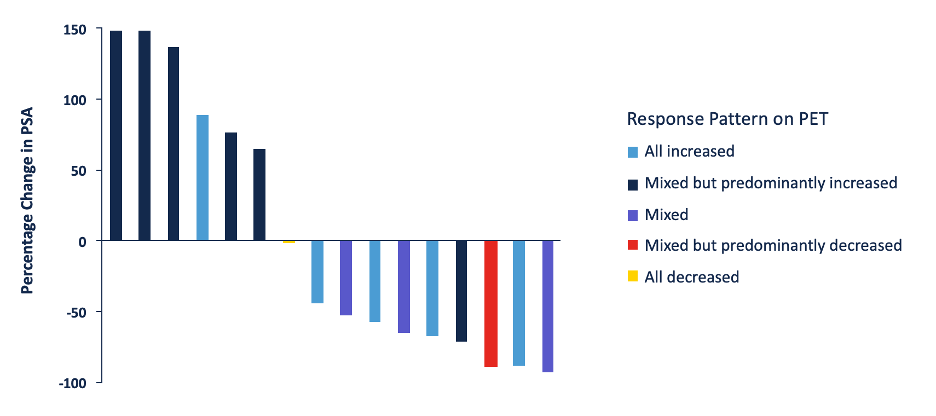
Men with a low the δ-percent SUVmax (DPSM) had a median time to therapy change of 12.2 months (95% CI, 11.3-NA) and a median overall survival of 37.2 months (95% CI, 28.9-NA), whereas those with a high DPSM had a median time to therapy change of 6.5 months (95% CI, 4.6-NA, p = 0.0001) and a median overall survival of 17.8 months (95% CI, 13.9-NA, p = 0.02). Men with a low the δ-absolute SUVmax (DASM) had a median time to therapy change of 12.2 months (95% CI, 11.3-NA) and a median overall survival of NA (95% CI, 37.2 months-NA), whereas those with a high DASM had a median time to therapy change of 6.9 months (95% CI, 6.1-NA, p = 0.003) and a median overall survival of 17.8 months (95% CI, 13.9-NA, p = 0.002). Time to therapy change stratified by DPSM and DASM is as follows: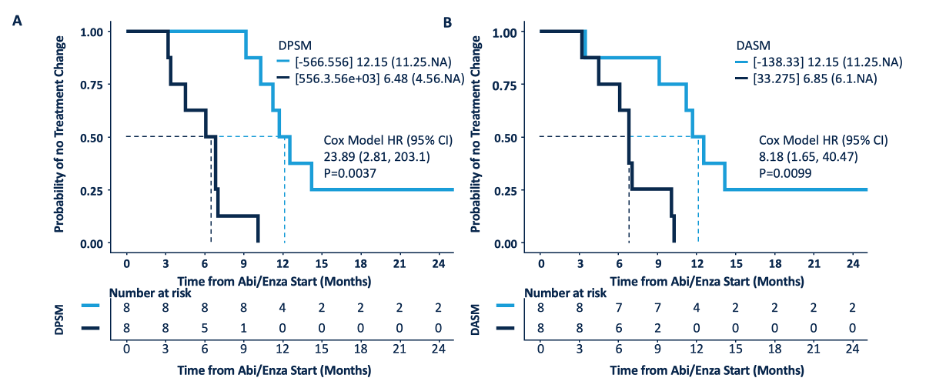
Overall survival stratified by DPSM and DASM is as follows: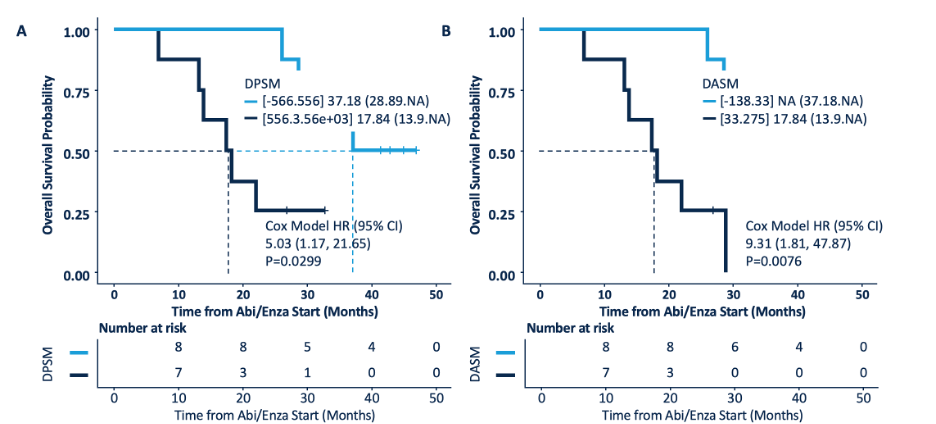
Bipolar androgen therapy is an emerging treatment for metastatic castration-resistant prostate cancer, but how it affects 18F-DCFPyL PET/CT images has only recently been evaluated.10 In a study of six men undergoing bipolar androgen therapy that received a PSMA PET/CT at baseline and 3 months after treatment, three patients had progression on imaging. Early progression was detected earlier for these patients with PSMA PET/CT compared to conventional imaging: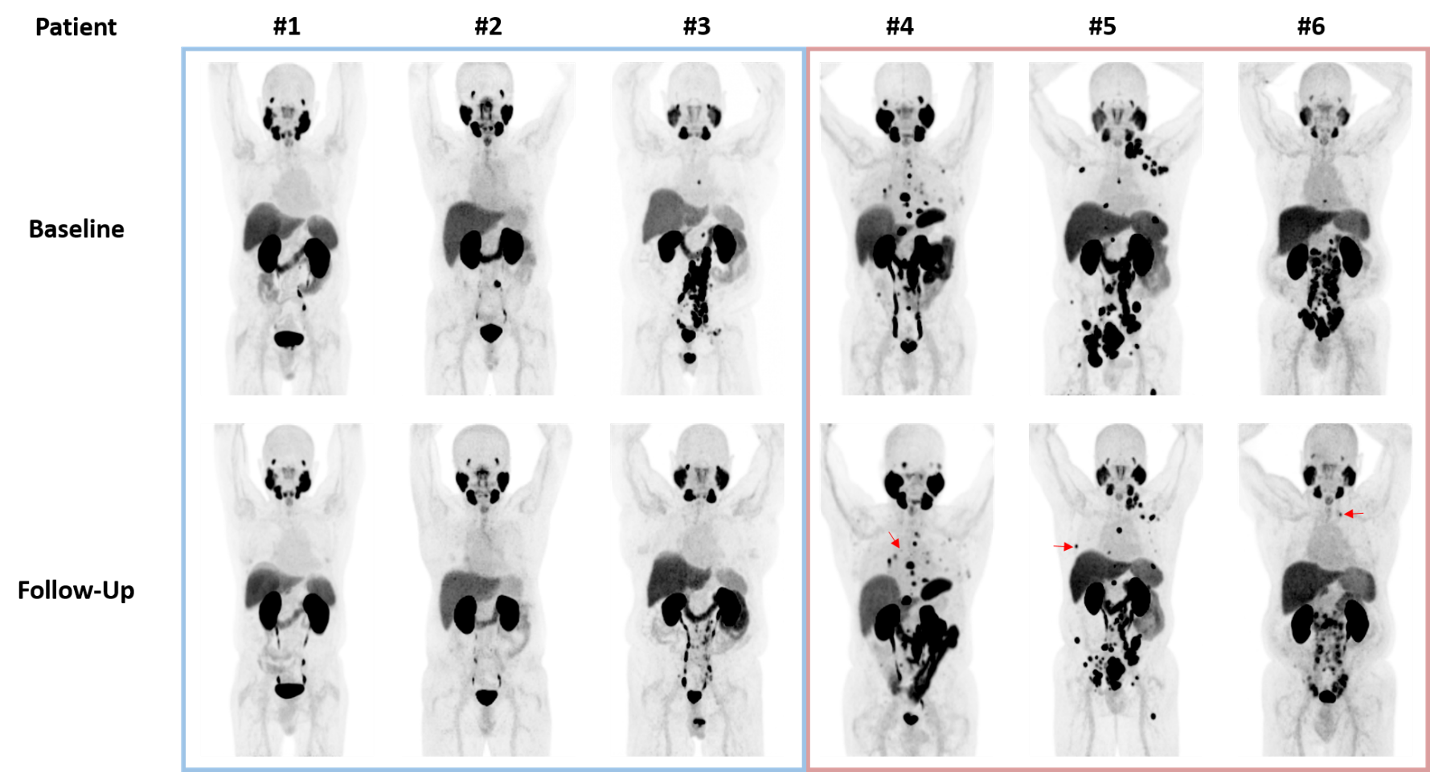
Several new and emerging fields for PSMA PET/CT include:
- Radiomics:
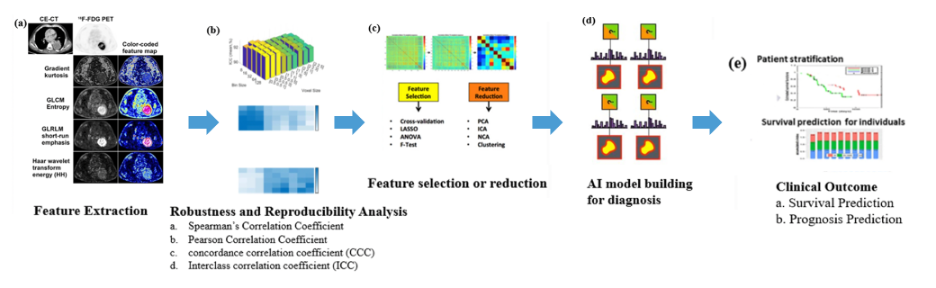
- Artificial intelligence: in the near term, Dr. Rowe states that we can expect artificial intelligence to “level us up” by providing (i) lesion classification, (ii) whole body tumor burden assessments, and (iii) prognostication and decision making based on scan findings and clinical data. Dr. Rowe suggests that this may also be extended to PSMA-RADS-AI:
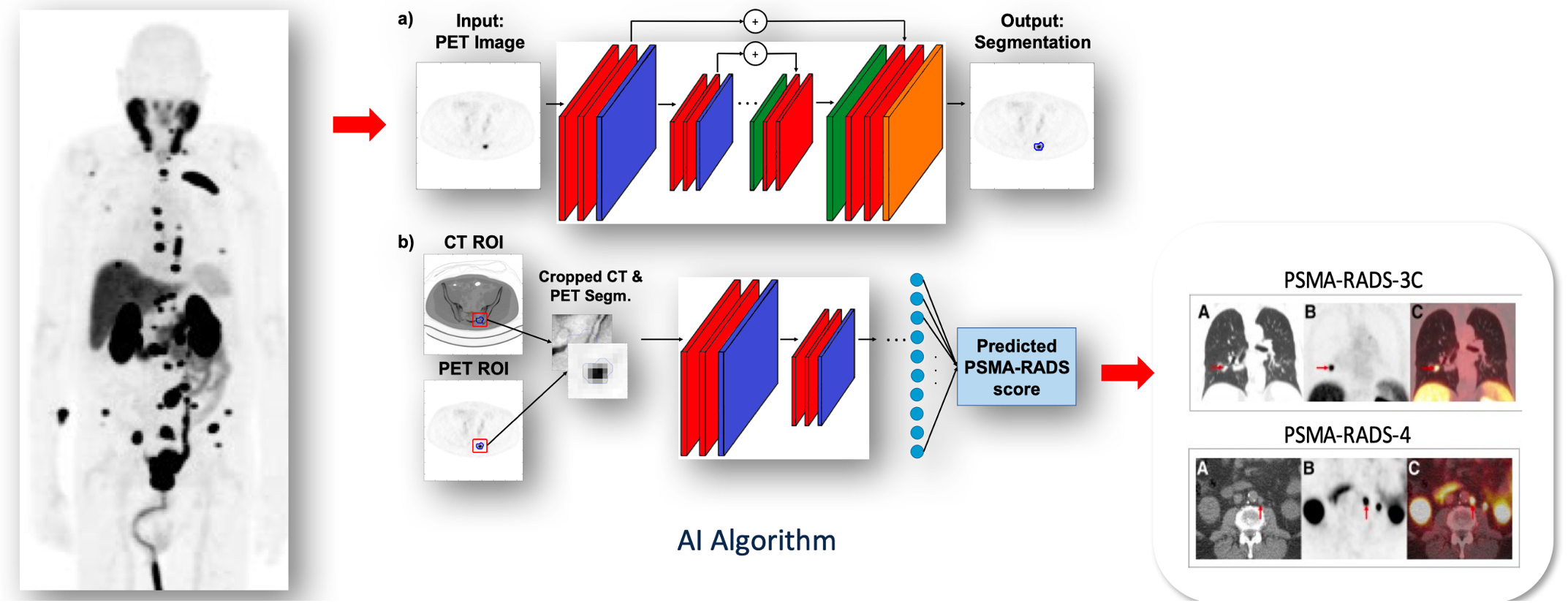
Dr. Rowe concluded his presentation by discussing registrational trials for PSMA PET with the following take home messages:
- There are already multiple indications for diagnostic PSMA-based imaging
- We are just starting to understand PSMA-targeted PET findings as imaging biomarkers
- Artificial intelligence will play an important role in biomarker development. However, only limited aspects of radiomics appear applicable to PET
Presented by: Steven P. Rowe, MD, PhD, University of North Carolina School of Medicine, Chapel Hill, North Carolina
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) Annual Meeting, Toronto, Ontario, Canada, Sat, June 8 – Tues, June 11, 2024
References:
- Pienta KJ, Gorin MA, Rowe SP, et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol. 2021 Jul;206(1):52-61.
- Hope TA, Eiber M, Armstrong WR, et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol. 2021 Nov 1;7(11):1635-1642.
- Surasi DS, Eiber M, Maurer T, et al. Diagnostic performance and safety of positron emission tomography with 18F-rhPSMA-7.3 in patients with newly diagnosed unfavorable intermediate- to very-high-risk prostate cancer: Results from a Phase 3, Prospective, Multicenter Study (LIGHTHOUSE). Eur Urol. 2023 Oct;84(4):361-370.
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021 Jul 1;27(13):3674-3682.
- Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol 2019 Jun 1;5(6):856-863.
- Fleming MT, Hermsen R, Purysko AS, et al. True-Positive 18F-Flutofolastat Lesions in Patients with Prostate Cancer Recurrence with Baseline-Negative Conventional Imaging: Results from the Prospective, Phase 3, Multicenter SPOTLIGHT Study. J Nucl Med. 2024 May 23 [Epub ahead of print].
- Sadaghiani MS, Sheikhbahaei S, Werner RA, et al. A systematic review and meta-analysis of the effectiveness and toxicities of Lutetium-177-labeled Prostate-specific Membrane Antigen-targeted Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer. Eur Urol. 2021 Jul;80(1):82-94.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Zukotynski KA, Emmenegger U, Hotte S, et al. Prospective, single-arm trial evaluating changes in uptake patterns on Prostate-Specific Membrane Antigen-Targeted 18F-DCFPyL PET/CT in patients with Castration Resistant Prostate Cancer Starting Abiraterone or Enzalutamide. J Nucl Med. 2021 Oct;62(10):1430-1437.
- Markowski MC, Velho PI, Eisenberger MA, et al. Detection of early progression with 18F-DCFPyL PET/CT in men with metastatic castration-resistant prostate cancer receiving bipolar androgen therapy. J Nucl Med. 2021 Sep 1;62(9):1270-1273.


