(UroToday.com) The 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) annual meeting featured a session on prostate cancer, and a presentation by Dr. Louise Emmett discussing response assessment in radiopharmaceutical therapies for prostate cancer. Dr. Emmett started her presentation by noting that for patients with high PSMA SUVmean (quartile 4), PSA50 response rate to 177Lu PSMA is excellent (89%). However, for those patients with low SUVmean (quartile 1) there is relatively poor 177Lu PSMA PSA50 response rates (29%), but which are reasonable with cabazitaxel (43%):

Dr. Emmett notes that screening imaging is only one part of the puzzle. In 2021, Gafita and colleagues1 published a nomogram of baseline characteristics for prediction of overall survival following 177Lu-PSMA-617. Among 270 patients, predictors included in the nomograms were time since initial diagnosis of prostate cancer, chemotherapy status, baseline hemoglobin, and 68Ga-PSMA-11 PET/CT parameters (molecular imaging TNM classification and tumor burden). The C-index of the overall survival model was 0.71 (95% CI 0.69-0.73):
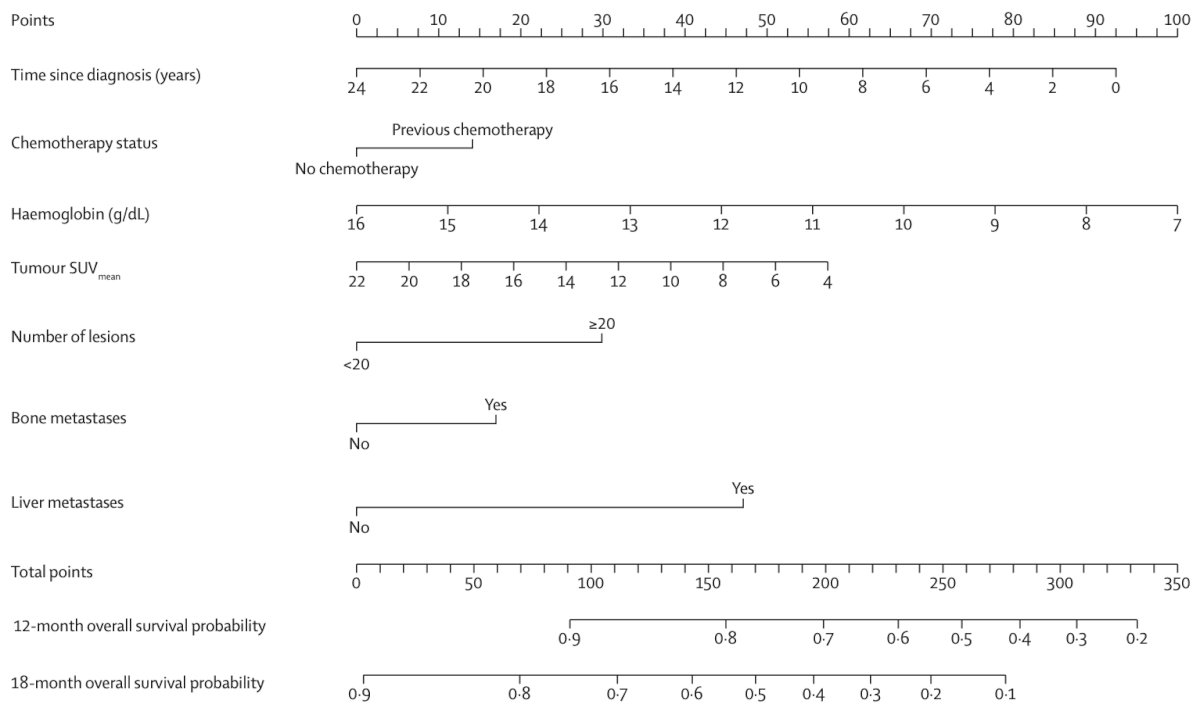
which was similar to the c-index of the PSA-progression-free survival model (0.70, 95% CI 0.68-0.72). Compared with high-risk patients, low-risk patients had significantly longer overall survival in the validation cohort (24.9 months vs 7.4 months; p < 0.0001) and PSA progression free survival (6.6 months vs 2.5 months; p = 0.022).
Radiation induced cell death includes direct and indirect DNA damage, leading to single strand and double strand breaks, with bystander effects known as the abscopal effect. However, the mechanism of cell death is complicated and predicting radiation sensitivity is complex. What we need is biomarkers to guide treatment. Dr. Emmett notes the following:
- PSA response and RECIST/PCWG3 criteria: PSA progression is considered unreliable early in treatment secondary to a flare, and PCWG3 requires confirmation (2 + 2) for disease progression, which can take months
- In TheraP,2 55% of patients demonstrated PSA progression prior to the third dose (week 12)
- In VISION,3 30% of patients did not have a significant response to treatment
- Better dynamic biomarkers will allow personalization of treatment and improved patient outcomes
With regards to PSMA PET/CT for response assessment using 177Lu-PSMA-617, Gafita and colleagues4 developed version 1.0 of a novel framework for response evaluation criteria in PSMA PET/CT and a composite response classification that combines responses by PSA measurements and by RECIP 1.0 (PSA + RECIP). RECIP is defined as follows:
- PSMA PET Partial Response: >30% decrease in PSMA volume without appearance of new lesions
- PSMA PET Progressive Disease: >20% increase in PSMA volume with appearance of new lesions
- PSMA PET Stable Disease: all other
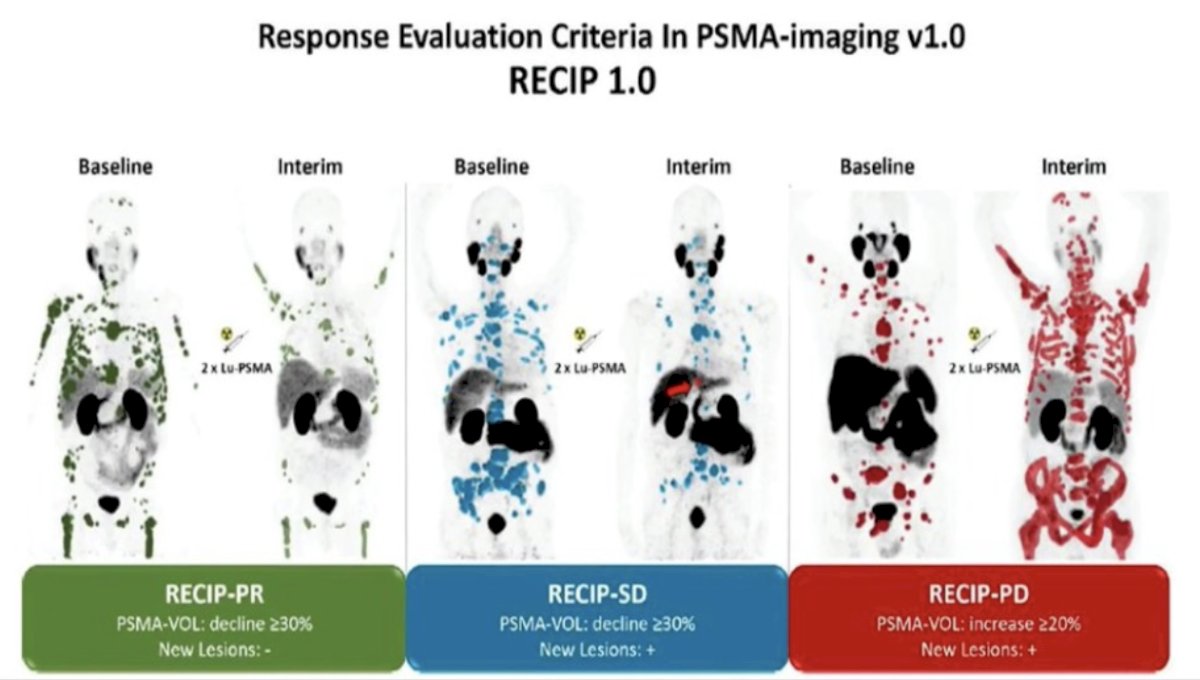
Overall, patients with RECIP progressive disease (n = 39; 8.3 months) had a shorter overall survival than patients with RECIP stable disease (n = 47; 13.1 months; p < 0.001) or RECIP partial response (n = 38; 21.7 months; p < 0.001).
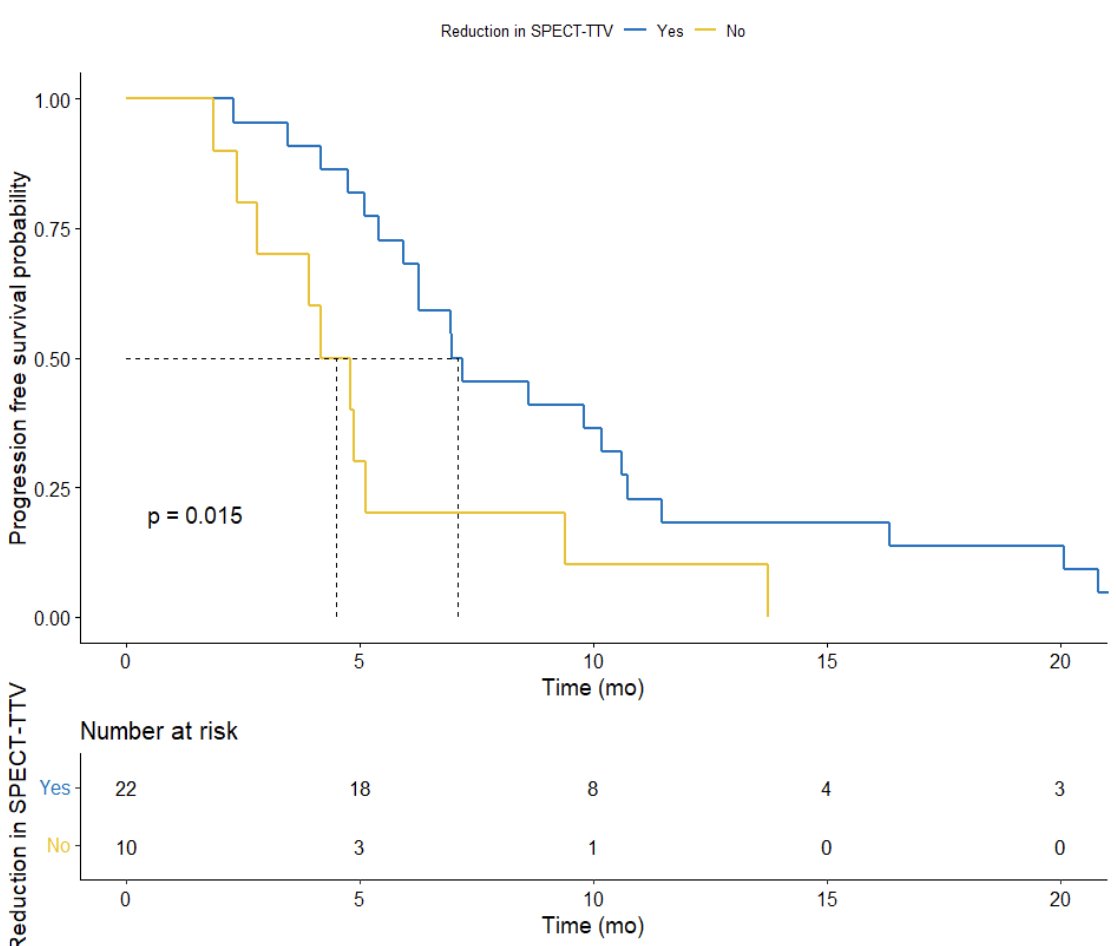
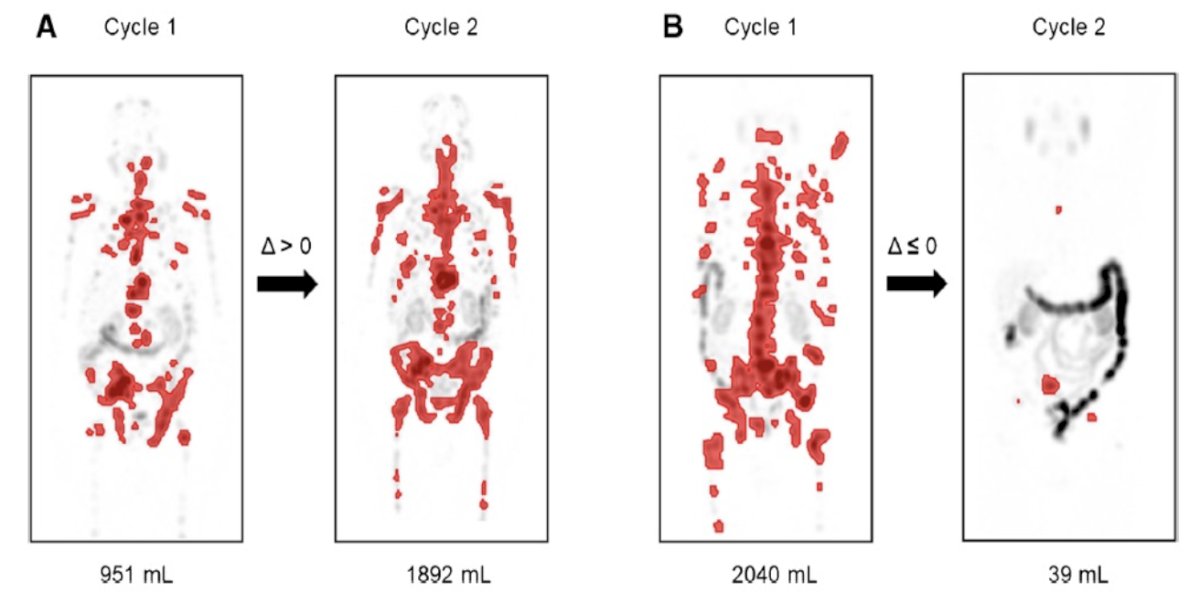
Previously, Dr. Emmett’s group investigated the predictive value of serial 177Lu-PSMA-617 SPECT/CT imaging in monitoring treatment response.5 Among 56 men, 32 had serial 177Lu-PSMA-617 SPECT/CT imaging at both cycle 1 and cycle 3, of which the median PSA progression-free survival was 6.3 months (95% CI, 5-10 months), median overall survival was 12.3 months (95% CI, 12-24 months), and the PSA50 response rate was 63%. Additionally, 177Lu-PSMA-617 SPECT/CT total tumor volume was reduced in 68% (median -0.20 m,3 95% CI, -1.4 to -0.001) and increased in 31% (median 0.36, 95% CI, 0.1-1.4). Any increase in 177Lu-PSMA-617 SPECT/CT total tumor volume was associated with shorter PSA progression free survival (HR 4.1, 95% CI 1.5-11.2; p = 0.006):
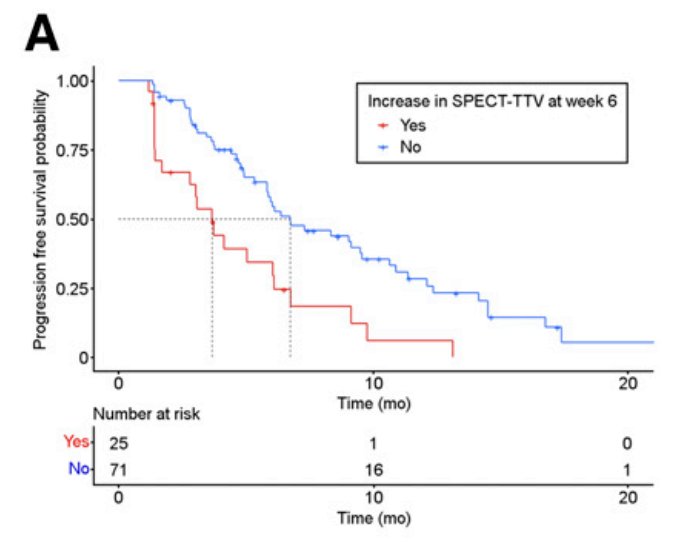
Dr. Emmett’s group subsequently published that 177Lu-PSMA SPECT quantitation at 6 weeks (corresponding with dose 2) predicts shorter progression-free survival.6 Among 127 men with progressive metastatic castration resistant prostate cancer, a PSA50 response was noted in 58% of patients, as well as a median progression free survival of 6.1 months (95% CI 5.5-6.7), and overall survival of 16.8 months (95% CI 13.5-20.1). Furthermore, median PSA progression free survival in those with an increase in 177Lu-PSMA SPECT total tumor volume was 3.7 months (95% CI, 2.8-6.8), compared with 6.7 months (95% CI, 5.8-10.6) in those with no increase in total tumor volume:
Another study by Neubauer et al.8 looked to assess tumor response with quantitated SPECT/CT and to correlate it with clinical outcome in metastatic castration-resistant prostate cancer patients treated with 177Lutetium-PSMA I&T therapy. This was a single center, observational study, of which 73 patients received 177Lu-PSMA I&T in metastatic castration resistant prostate cancer patients treated with at least two cycles of 177Lu-PSMA I&T 6 times weekly. After the first and second cycle, quantitated SPECT/CT was acquired 48 hours after injection:
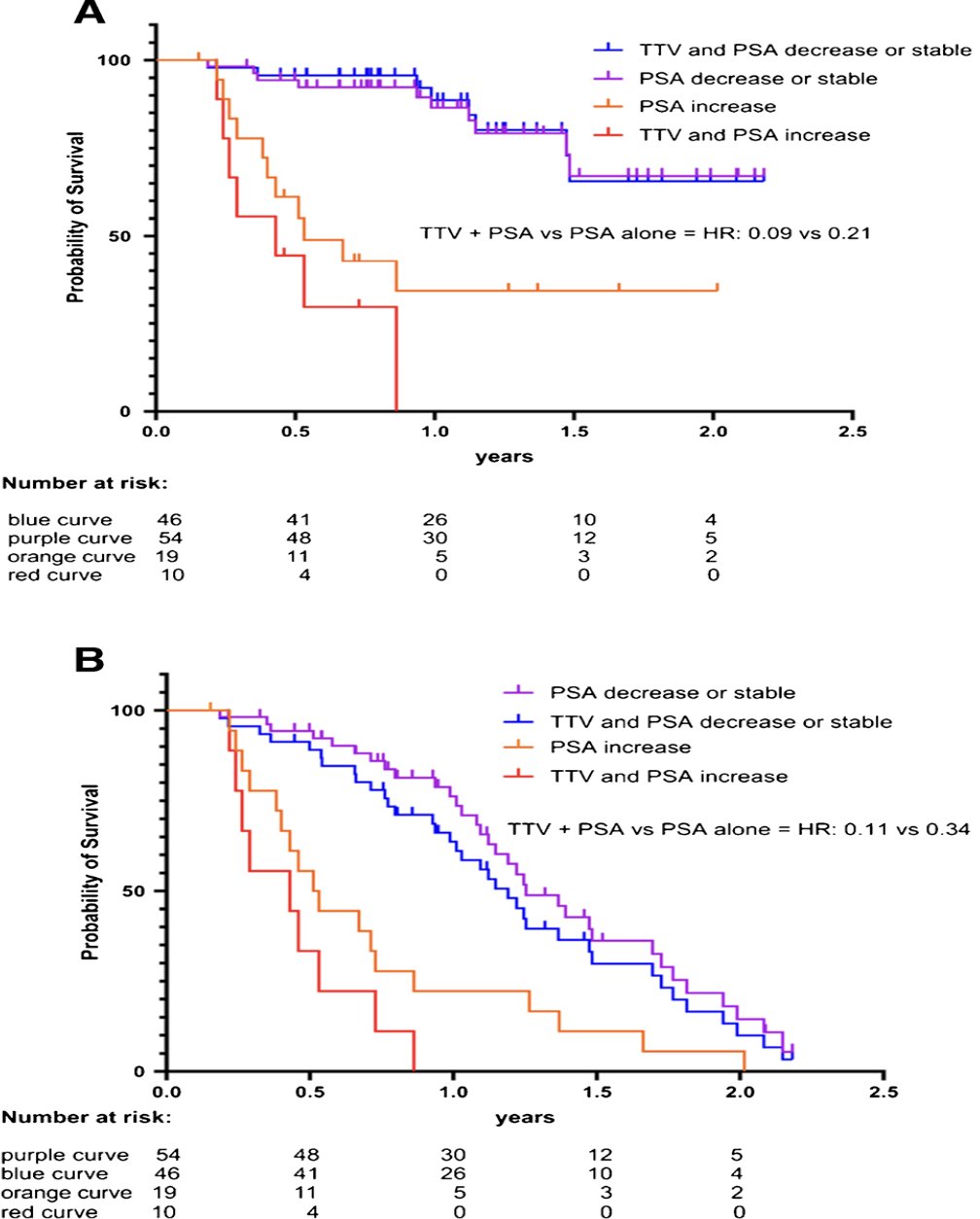
The median follow-up was 8.9 months (range 1.4-26.6 months). Stable or decreased total tumor volume at cycle 2 was associated with longer overall survival (HR 0.28, 95% CI 0.09-0.86, p < 0.01). Similar, stable, or decreased PSA was associated with longer overall survival (HR 0.21, 95% CI 0.07-0.62, p < 0.01) and PSA progression free survival (HR 0.34, 95% CI 0.16-0.72, p < 0.01). Combining total tumor volume and PSA resulted in an augmented prognostic value for overall survival (HR 0.09, 95% CI 0.01-0.63; p < 0.01) and for PSA-progression free survival (HR 0.11, CI 0.02-0.68; p < 0.01). A reduction of SUVmax or SUVmean was not prognostically relevant for either overall survival (p 0.88 and 0.7) or PSA-progression free survival (p 0.73 and 0.62, respectively):
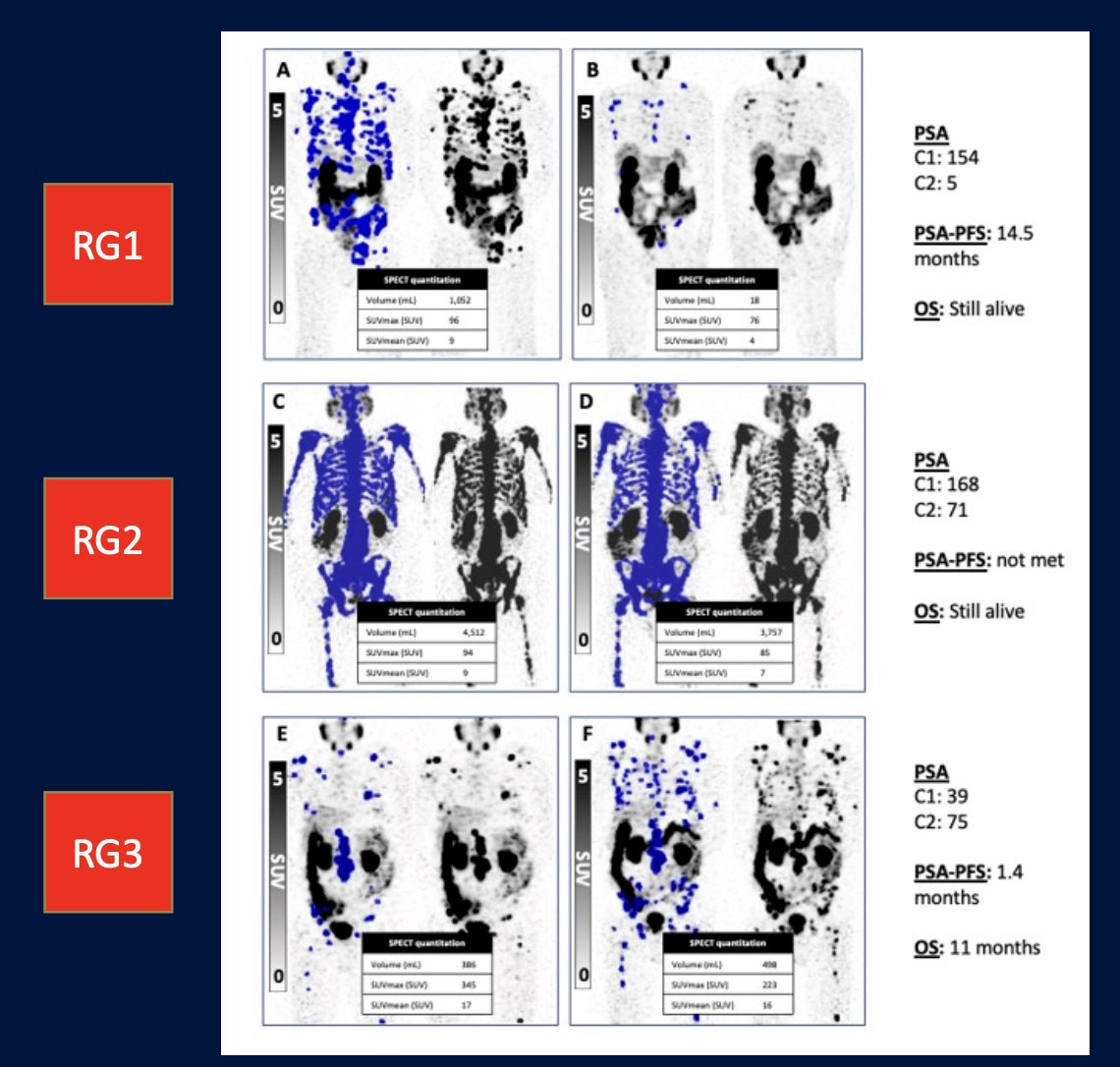
In 2023, Dr. Emmett and colleagues reported results of their study evaluating progression free survival and overall survival based on treatment interval adjustment utilizing 177LuPSMA 24-hour SPECT/CT and early PSA response.8 There were 125 men treated with 6 times weekly 177LuPSMA-I&T doses (median 3 cycles, IQR 2-4) at a median dose of 8.0 GBq (95% CI 7.5-8.0). Following dose 2 (week 6), a composite PSA and 177LuPSMA 24-hour SPECT/CT imaging response determined ongoing management:
- Response Group 1 (marked reduction in PSA/imaging partial response): break in treatment until subsequent PSA rise then re-treatment
- Response Group 2 (stable or reduced PSA and/or imaging stable disease): 6-weekly treatments until six doses, or no longer clinically benefiting
- Response Group 3 (rise in PSA and/or imaging progressive disease): recommend for alternative treatment
The results were as follows:
- Response Group 1: progression free survival 12.3 months, overall survival 22 months, treatment break for 6 months
- Response Group 2: progression free survival 6.2 months, overall survival 15 months, no treatment break
- Response Group 3: progression free survival 2.8 months, overall survival 11 months, early more to alternative treatment
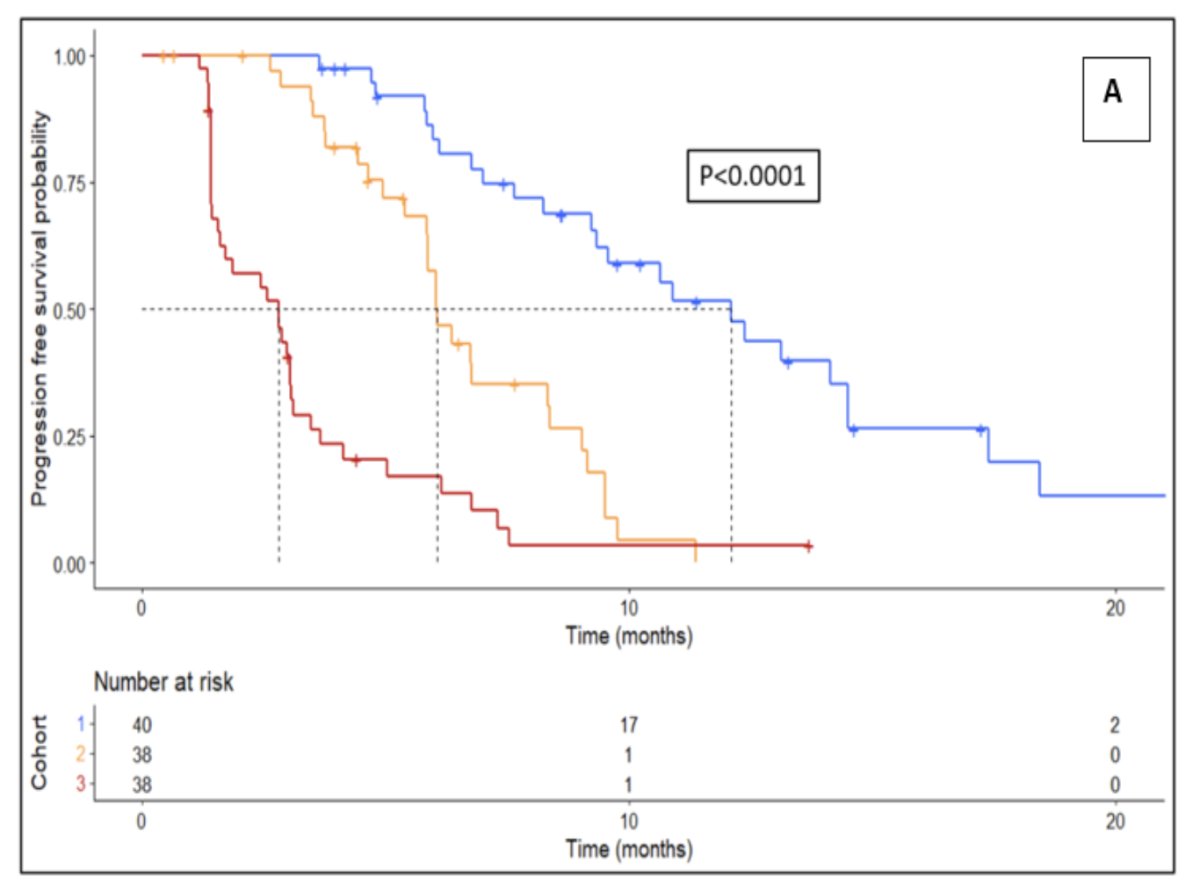
The progression free survival Kaplan Meier curve stratified by response group is as follows (p < 0.0001):
In 2023, the PROMISE criteria were proposed as a framework for whole body staging to describe the prostate cancer disease extent on PSMA PET.9 PSMA expression assessed by the PSMA-expression score was used in several trials, and a low PSMA-expression score is a negative prognosticator of overall survival after 177Lu-PSMA radioligand therapy. The following highlights the PROMISE V2 framework:
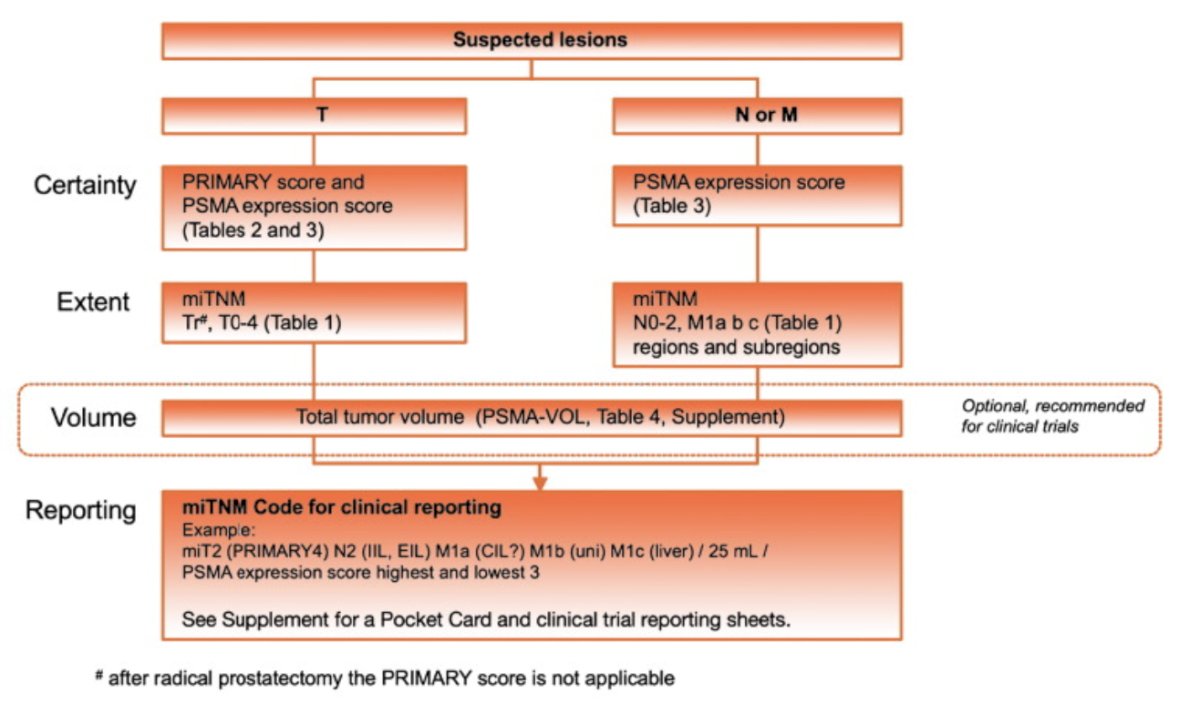
Dr. Emmett notes that there is a need to develop standardized criteria for treatment response to systemic therapies on PSMA PET/CT both for prospective trials and clinical purposes. A proposal for systemic therapy response assessment has been suggested to include the following:
- Appearance of 2 or more new PSMA positive distant lesions
- Appearance of 1 or new PSMA positive lesions plus consistent clinical or laboratory data and recommend confirmation by biopsy or correlative imaging within 3 months of PSMA PET
- Increase in size or PSMA uptake of 1 or more existing lesions by at least 30%, plus consistent clinical or laboratory data or confirmation by biopsy or correlative imaging within 3 months of PSMA PET
Dr. Emmett highlighted that there are treatment response criteria on the horizon:
- PCWG4:
- Prostate Cancer Working Group criteria for identification of progressive disease
- PSMA PET criteria, as well as diagnostic CT and bone scan for evaluation of systemic treatment in prostate cancer
- SPARC:
- Standardized PSMA PET analysis and reporting consensus
- Multidisciplinary expert consensus on optimal reporting standards
- Downloadable reporting templates
- Recommended minimum criteria
Dr. Emmett concluded her presentation by discussing response assessment in radiopharmaceutical therapies for prostate cancer with the following take home messages:
- There is increasing evidence to show prognostic value of dose two 177Lu PSMA SPECT/CT for PSA progression free survival and overall survival
- Effective early response biomarkers offer an opportunity for early intervention and improved personalization of treatment regimens for de-intensification/intensification
- Two biomarkers are better than one
- Change in intensity is not useful, as we need volume change or new lesions
- PCWG4 is nearly out, which will incorporate PSMA PET for assessment of treatment response in clinical trials
- SPARC represents expert clinical reporting guidelines
Presented by: Louise Emmett, MBChB, FRACP, MD, St. Vincent’s Hospital, Sydney, NSW, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Nuclear Medicine & Molecular Imaging (SNMMI) Annual Meeting, Toronto, Ontario, Canada, Sat, June 8 – Tues, June 11, 2024
References:
- Gafita A, Calais J, Grogan TR, et al. Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: An international, multi-centre, retrospective study. Lancet Oncol. 2021 Aug;22(8):1115-1125.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Gafita A, Rauscher I, Weber M, et al. Novel framework for treatment response evaluation using PSMA PET/CT in patients with metastatic castration-resistant prostate cancer (RECIP 1.0): An International multicenter study. J Nucl Med. 2022 Nov;63(11):1651-1658.
- Pathmanandavel S, Crumbaker M, Ho B, et al. Evaluation of 177Lu-PSMA-617 SPECT/CT quantitation as a response biomarker within a prospective 177Lu-PSMA-617 and NOX66 Combination Trial (LuPIN). J Nucl Med. 2023 Feb;64(2):221-226.
- John N, Pathmanandavel S, Crumbaker M, et al. 177Lu-PSMA SPECT Quantitation at 6 Weeks (Dose 2) Predicts Short Progression-Free Survival for Patients Undergoing 177Lu-PSMA-I&T Therapy. J Nucl Med. 2023 Mar;64(3):410-415.
- Neubauer MC, Nicolas GP, Bauman A, et al. Early response monitoring during [177Lu]Lu-PSMA I&T therapy with quantitated SPECT/CT predicts overall survival of mCRPC patients: Subgroup analysis of a Swiss-wide prospective registry study. Eur J Nucl Med Mole Imaging. 2024 Mar;51(4):1185-1193.
- Emmett L, John N, Pathmanandavel S, et al. Patient outcomes following a response biomarker-guided approach to treatment using 177Lu-PSMA-I&T in men with metastatic castrate-resistant prostate cancer (Re-SPECT). Ther Adv Med Oncol. 2023 Mar 1:15:17588359231156392.
- Seifert R, Emmett L, Rowe SP, et al. Second Version of the Prostate Cancer Molecular Imaging Standardized Evaluation Framework Including Response Evaluation for Clinical Trials (PROMISE V2). Eur Urol. 2023. May;83(5):405-412.


