The study population included adult men and women with either neurogenic bladder or overactive bladder symptoms who failed previous behavioral and/or pharmacological management. Participants’ perception of bladder condition was a primary outcome of the trial.
A total number of 48 subjects were enrolled in the study (28 participants with neurogenic bladder (64% female) and 20 with OAB (100% female)). Both study groups have received 30-minute sessions three times per week for the duration of 12 to 13 weeks. Participants were instructed to apply TTNS at home unilaterally. Those who received a sham device were told to place their unit to the lateral aspect of the lower leg. Researchers have checked a study blinding success by asking patients with TENS and sham devices about their perceived group assignment, which showed no correlation.
The study didn’t demonstrate a significant difference between active treatment and sham devices (Figure 1).
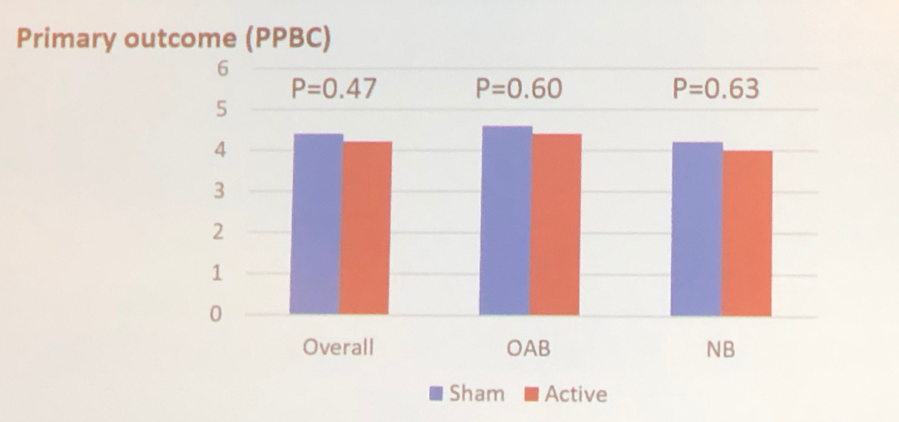
There was no significance in secondary patient-reported outcomes: urinary symptoms and NBSS score in neurogenic bladder patients.
According to Blayne Welk, MD there is a number of RCTs assessing TTNS effectiveness, but they are small, underpowered, and at high risk for bias. Recent data show that TTNS is non-inferior to PTNS, but more research is needed to standardize TTNS techniques.
Presented by: Blayne Welk, MD, MSc. Western University.
Written by: Hanna Stambakio, BS, Clinical Research Coordinator, Division of Urology, University of Pennsylvania, Twitter: @AStambakio at the Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction Winter Meeting, SUFU 2019, February 26 - March 2, 2019, Miami, Florida


