Dr. Tannir continued and discussed on the important task of how to better select patients for cytoreductive nephrectomy. We have some tools that we can use, which include preoperative factors, response to presurgical therapy, and mutational studies as biomarkers. Data have shown that increasing the number of preoperative risk factors is inversely correlated with survival.
Preoperative risk factor that were examined include low albumin, high LDH, liver metastases, symptoms at presentation, retroperitoneal lymph node involvement, supra-diaphragmatic lymph node involvement, and clinical T stage 3 or 4 (3). Other known preoperative risk criteria include the Heng criteria, which have shown a similar correlation. These include low hemoglobin, high calcium, high neutrophilic count, high platelet count, Kranofsky performance score less than 80%, and time from diagnosis to therapy of less than 1 year (4).
Response to presurgical therapy, such as sunitinib, of more than 10% has been shown to improve overall survival with a HR of 0.26 (0.08-0.089) (5).
Finally, genomic studies to identify who might benefit from cytoreductive nephrectomy have been performed. When patients were stratified by BAP1 and PBRM1 mutation status, the presence of mutation in these two genes were correlated with significant worse survival (6).
Next, Dr. Tannir discussed the CARMENA trial, which was a phase 3 randomized study comparing nephrectomy plus Sunitinib vs. Sunitinib alone in first line metastatic RCC (7). This trial clearly showed no difference between both arms in overall survival. However, this trial had significant limitations; including the fact that all patients were either intermediate risk (57%) or poor risk (43%). Furthermore, in the nephrectomy group 7.1% of the nephrectomy patients did not actually undergo nephrectomy, and 17.7% did not actually receive sunitinib. Moreover, in the sunitinib group, 4.9% never received sunitinib, and 17% underwent nephrectomy! It would seem that the patients that we were actually interested in ascertaining the effect of cytoreductive nephrectomy, were not included in the trial (favorable intermediate risk patients with oligometastasis only). Lastly, sunitinib is no longer relevant considering the findings showed by the Checkmate 214 study showing a clear advantage of immunotherapy and angiogenesis inhibitor combination.
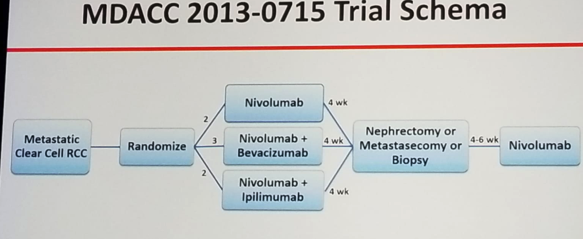
Dr. Tannir concluded his talk and summarized his views on this topic. According to him, CARMENA does not support use of cytoreductive nephrectomy in the setting of sunitinib. However, this trial is no longer relevant with the approval of Nivolumab/ipilimumab and soon other immunotherapy and TKI regimens in first line metastatic RCC. Novel clinical trials are needed to replace CARMENA. An example for such a trial is the MDACC 2013-0715 trial (NCT 02210117) which randomizes metastatic clear cell RCC patients to 3 arms: 1. Nivolumab, 2. Nivolumab + Bevacizumab, 3. Nivolumab + ipilimumab. All treatments will be given for a duration of 4 weeks and then all patients will undergo nephrectomy or metastasectomy or biopsy. Lastly, all will receive 4-6 weeks of nivolumab (study design in figure 1).
Figure 1
In summary, upfront cytoreductive nephrectomy does not currently have any supportive evidence, and it should only be considered in patients with intermediate risk and solitary metastasis, or oligometastasis in one organ, or in intermediate risk disease with IVC thrombus. There is a mechanistic rationale for keeping the primary renal tumor in situ to permit priming and training of effector T cell populations. In an appropriately selected patient, a deferred nephrectomy can be incorporated in an overall debulking strategy once systemic therapy has been shown to be effective. We need to assess the ability of newer systemic therapies in producing a complete response, and whether cytoreductive nephrectomy would even be needed in either setting. The benefit of cytoreductive nephrectomy is inversely associated with the development of more efficacious systemic therapies, and it should be tailored to specific scenarios.
Presented By: Nizar Tannir, MD Anderson Cancer Center
References:
Mickisch GH et al. Lancet 2007
Flanigan RC et al. J Urol 2004
Culp SH et al. Cancer 2010
Heng DY Eur Urol 2014
Abel EJ et al. EUr Urol 2011
Manley BJ AUA 2016 annual meeting
Méjean A et al. NEJM 2018
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow, SUO, University of Toronto, Princess Margaret Cancer Centre, @GoldbergHanan, at the 19th Annual Meeting of the Society of Urologic Oncology (SUO), November 28-30, 2018 – Phoenix, Arizona


