Both the AUA and NCCN guidelines are quite heterogeneous in their recommendations for follow-up:
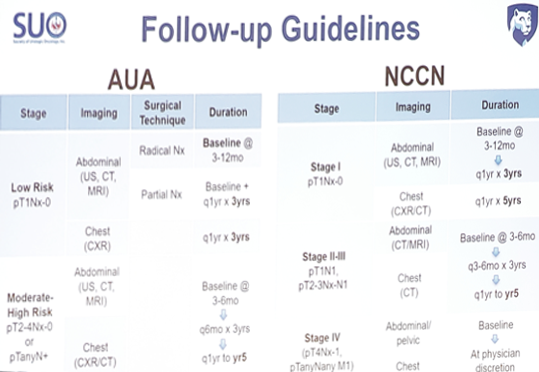
The effectiveness of guideline recommendations has also been called into question.1 When the guidelines are strictly followed, both the AUA and NCCN (2014 schedules) missed 1/3rd of all recurrences. The reasons for missed recurrences include (i) basic risk stratification (stage only), and (ii) a simplistic method for follow-up duration (cumulative incidence of recurrence). The AUA and NCCN follow-up schedules fail to account for:
- Dynamic changes in recurrence risk over time.
- Influence of competing risks from comorbid conditions.
- RCC recurrences do occur beyond 5 years – 4% local recurrences, 12% distant recurrences.
Weibull modeling captures dynamic interaction between the risk of RCC recurrence (stratified by stage and relapse location) and risk of non-RCC death (stratified by age and Charlson Comorbidity index).2 This modeling approach also detects when the focus of health maintenance could shift away from identifying recurrence. Thus, the transition time point would equal duration of follow-up – when the competing health risks (non-RCC death) exceed the risk of RCC recurrence.
A similar concept, but a new model was presented by Dr. Merrill’s group at the 2019 AUA. They identified 1,672 patients who underwent surgery for M0 RCC between 1999-2018. Patients were stratified by pathologic stage (pT1aN0-x, pT1bN0-x, pT2N0-x, pT3aN0-x, pT3b/c/4N0-x), histology (clear cell, papillary and chromophobe), age, and ECOG status (0,1,2-4). Surveillance duration was estimated as the time point in which the cumulative incidence of non-RCC death exceeded that of RCC recurrence for a given stage, histology, age and ECOG score. At a median follow-up of 2.1 years (IQR 0.6-5.1 years), a total of 272 (16.3%) recurrences and 234 (14.0%) non-RCC deaths had occurred. For patients age 50 with pT1aN0-x clear cell and ECOG 0, the incidence of non-RCC death exceeded that of recurrence after 4.4 years. However, when ECOG status was 1 or 2-4 or the patient age 70, regardless of ECOG status, the incidence of non-RCC death exceeded that of recurrence at 30 days following surgery, suggesting that routine oncologic surveillance may not be necessary. Alternatively, for patients age 50 with >=pT3aN0-x clear cell, regardless of ECOG status, the incidence of non-RCC death failed to exceed that of recurrence for >15 years, suggesting longer surveillance than currently recommended.
Modeling competing for risks to define follow-up duration provides an opportunity to be more selective and provide better stewardship of medical resources. It also may improve equity and reduce the heterogeneity of care, given that a strong driver of surveillance intensity in practice is age. Furthermore, it is important to separate pT1a from pT1b, since pT1a is less aggressive (incidence of pulmonary metastasis is rare: 0.005-0.09%). Thus, there should be less chest/abdominal imaging for pT1a tumor follow-up. For patients with high risk RCC (>= pT3a), 1/5th of initial recurrences are outside of the recommended imaging templates (chest/abdomen), including 7% in the pelvis, 6% in the brain, and 11% in bone, other sites. Thus, more comprehensive imaging may be necessary for these high-risk patients. Symptomatic recurrences are associated with a 3-fold increased risk of overall and disease-specific mortality.
Dr. Merrill concluded with several next steps that will improve RCC follow-up optimization:
- Strong rationale supporting optimization – both from the physician and patient perspective
- Alternative risk stratification schemas and novel models are available
- The AUA guidelines, published in 2013, need evidence-based updating
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 20th Annual Meeting of the Society of Urologic Oncology (SUO), December 4 - 6, 2019, Washington, DC
References:
- Stewart SB, Thompson RH, Psutka SP, et al. Evaluation of the National Comprehensive Cancer Network and American Urological Association renal cell carcinoma surveillance guidelines. J Clin Oncol 2014 Dec 20;32(36):4059-4065.
- Stewart-Merrill SB, Thompson RH, Boorjian SA, et al. Oncologic surveillance after surgical resection for renal cell carcinoma: A novel risk-based approach. J Clin Oncol 2015;33(35):4151-4157.


