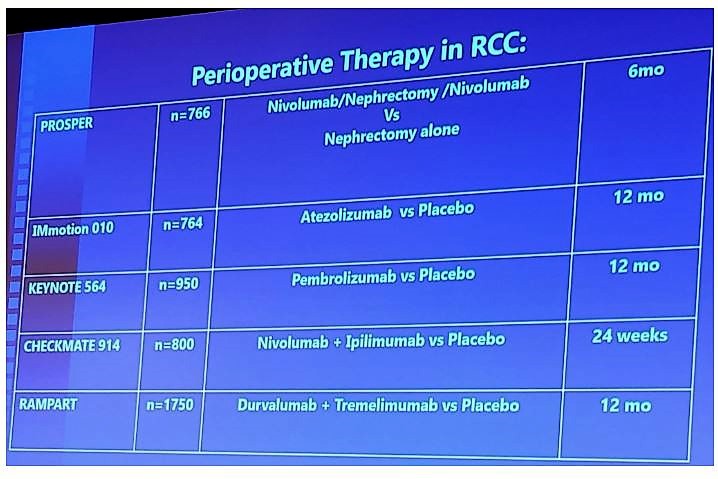
Washington, DC (UroToday.com) As part of the SUO-CTC clinical trials session, Dr. Gennady Bratslavsky provided an important update on adjuvant trials for renal cell carcinoma (RCC) within the CTC. As we are aware, there have been no clinical trials that have provided an overall survival benefit, with only S-TRAC providing a disease-free survival benefit,1 Currently, there are exciting perioperative trials in RCC, focusing on immunotherapy options:
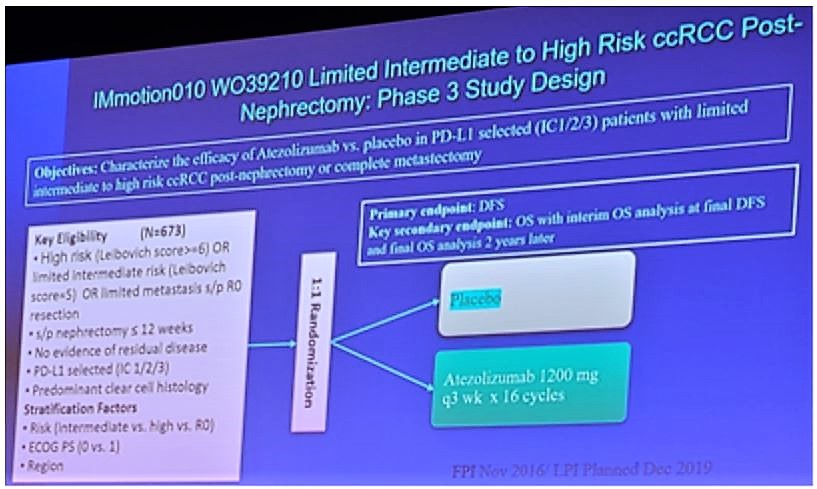
The IMmotion 010 trial is a phase 3 trial assessing limited intermediate to high-risk clear cell renal cell carcinoma post-nephrectomy. The objective is to characterize the efficacy of atezolizumab versus placebo in PD-L1 selected (ICI 2/3) patients with limited intermediate to high-risk clear cell RCC post-nephrectomy or complete metastasectomy. 673 patients are being randomized 1:1 to either placebo or atezolizumab 1200 mg q3 weeks x 16 cycles. The primary endpoint is DFS and key secondary endpoints are OS with interim OS analysis at final DFS and final OS analysis two years later. A schema for the trial is as follows:
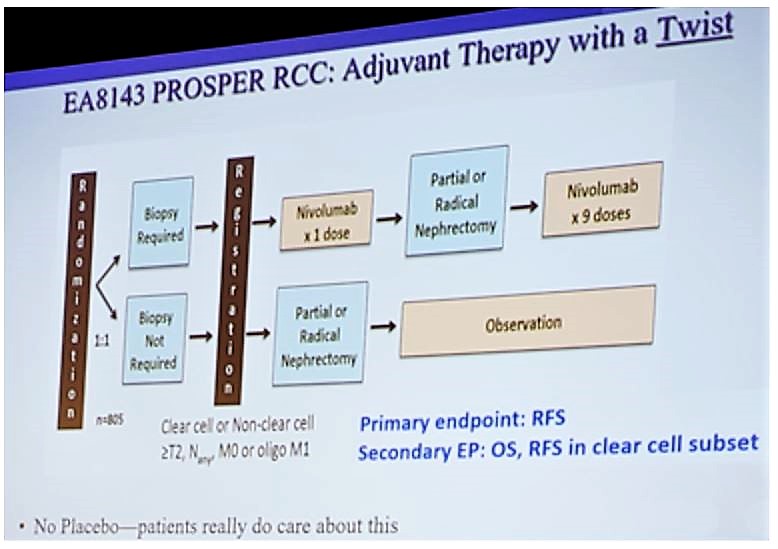
A second key trial is the EA8143 PROSPER adjuvant trial. This trial will randomize patients 1:1 to biopsy vs no biopsy, and then registration occurs before commencing nivolumab (1 dose), followed by partial/radical nephrectomy, followed by nivolumab (9 doses); OR partial/radical nephrectomy followed by observation. The primary endpoint is recurrence-free survival (RFS) and key secondary endpoints include OS and RFS in the clear cell subset. The trial schema for PROSPER is as follows:
The SUO-CTC has many opportunities, including imaging, biomarkers, adjuvant trials, neoadjuvant trials, and technology assessment trials. Organ-specific opportunities include:
- Kidney: adjuvant therapy, advanced disease
- Bladder: NMIBC, upper tract disease
- Prostate: oral anti-androgens, active surveillance
Presented by: Gennady Bratslavsky, MD, Professor, and Chair of Urology, Director of Prostate Cancer Program, Division Chief of Urologic Male Health and
Department Chief of Urologic Oncology, Upstate Medical Center, Syracuse, New York
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md, at the 20th Annual Meeting of the Society of Urologic Oncology (SUO), December 4 - 6, 2019, Washington, DC
References:
1. Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med 2016;375(23):2246-2254.


