For this study, gene expression data of 17,967 prospective radical prostatectomy samples generated from Decipher testing (GenomeDx Biosciences) and 1,135 retrospective samples with long term follow up were used in these analyses. All samples were from formalin-fixed paraffin-embedded tissues and the microarray was used for gene expression profiling. Associations between expression of PSMA and Axumin transporter genes (LAT1-4 and ASCT1-2) and pathologic variables, PAM50 molecular subtypes, and clinical outcomes were assessed. Multivariable analysis was performed to compare metastasis-free survival and lymph node involvement.
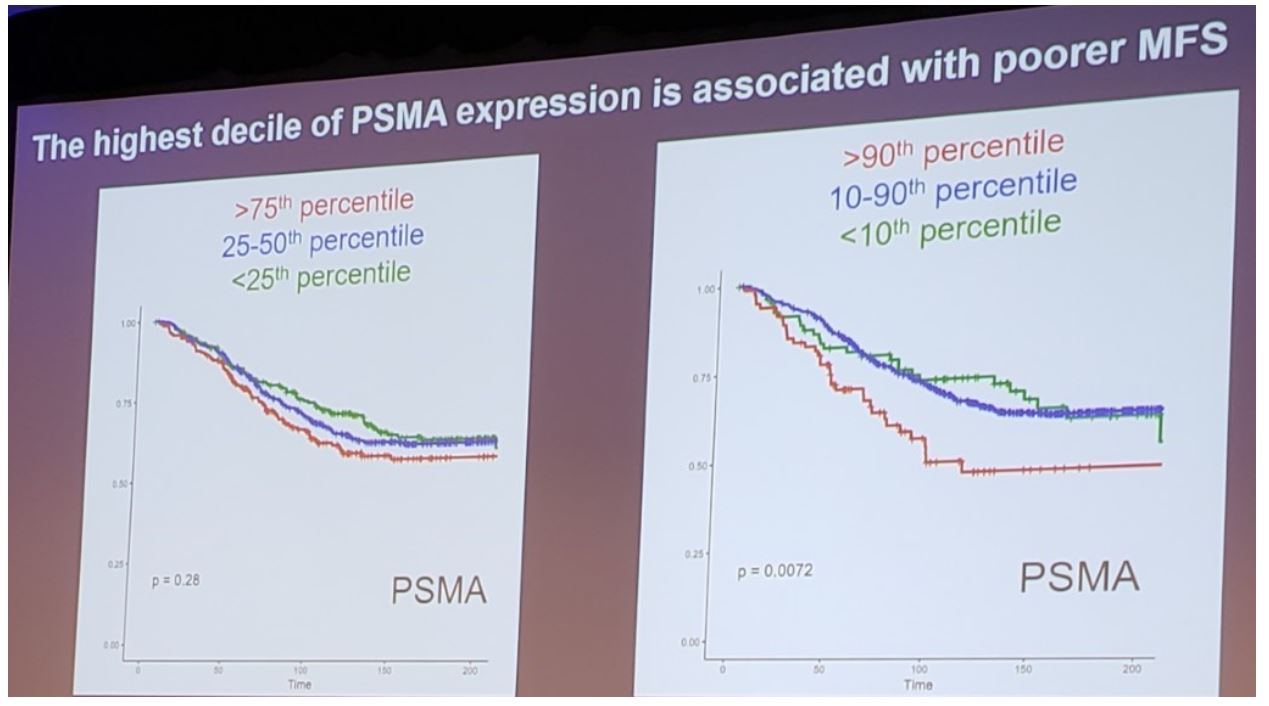
In the radical prostatectomy specimens, all Axumin transporter genes (LAT 1-4, ASCT1-2) were expressed at lower levels when compared with PSMA (IQR -0.1-0.8 versus IQR 1.4-2.6, p <0.0001). LAT2 and ASCT2 were more highly expressed than other Axumin transporters (p<0.0001). While PSMA expression was positively correlated with metastatic genomic risk (Decipher), and pathologic Gleason score (p<0.0001 for both), LAT2, LAT3, and ASCT2 were inversely correlated with metastatic genomic risk in primary tumors (p<0.0001), and less expressed in Gleason score 9-10 tumors (p<0.0001 for all). In multivariable analysis adjusting for Gleason grade, stage, PSA at diagnosis, and lymph node involvement, high PSMA expression remained independently prognostic of MFS (HR 1.3, p=0.028) but did not reach significance for lymph node involvement (HR 1.3, p=0.09). After multivariable analysis, only LAT3 expression was prognostic of improved MFS (HR 0.66, p<0.0001). Additionally, the highest decile of PSMA expression is associated with poor MFS:
With regard to molecular subtypes, Luminal B subtype was notable for overexpression of PSMA, while Luminal A was highly expressed in ASCT2 and LAT2 (p<0.0001). Basal subtype was notable for lowest expression of ASCT1 and LAT3 (p<0.0001). Although PSMA expression did not correlate with ERG positive or negative prostate cancers, LAT2, ASCT1, and ASCT2 were overexpressed in ERG negative tumors (p<0.0001 for all).
Dr. Chu concluded her presentation of this novel work assessing expression of PSMA and Axumin transporter genes with the following take-home messages:
- In radical prostatectomy specimens, PSMA expression is positively correlated with genomic risk scores and predictive of poorer metastasis-free survival
- After multivariate analysis, LAT3 expression was inversely correlated to metastatic risk
- Molecular subtypes of prostate cancer variably express PSMA and Axumin transporter genes
- While these findings suggest that gene expression analysis may have utility in selecting prostate cancer patients for different imaging modalities, further studies are necessary to validate the prognostic value of these imaging targets
- Additional studies into the role of these targets in underlying tumor biology and metastatic disease are also warranted.
1. Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol 2017;71(4):630-642.


