(UroToday.com) The annual Young Urologic Oncologists (YUO) program featured the top two outstanding poster presentations, highlighted by full presentations. The first presentation was by Dr. Neil Mendhiratta, PGY-5 resident at the UCLA Department of Urology, entitled “Decisional Regret and Financial Toxicity Among Patients with Benign Renal Masses.” Treatment of benign renal masses may often be unnecessary yet can lead to significant costs, morbidity, and mortality. Indeed, the cost of benign tumor resections includes $55,000 per hospitalization and $153 million annually. In this population, individual burdens such as decisional regret and financial costs associated with treatment are not well understood. The aim of this study was to assess decisional regret and financial toxicity among patients diagnosed with benign renal masses and to identify patient and tumor-associated predictors of each.
For this study, 70 members of a support group who had been diagnosed with benign renal tumors and who also reside in the United States were surveyed. In addition to demographic and clinical information, the survey evaluated decisional regret, using the modified decisional regret scale, and financial toxicity, using the COmprehensive Score for financial Toxicity (COST). Predictors of decisional regret (DRS score > 25) and financial toxicity were identified using univariate and multivariate logistic and linear regression analyses.
A total of 70 subjects provided complete demographic, clinical, and questionnaire data. The mean age was 48.0 +/- 12.6 years, 89% of patients were female, and 86% of patients were white. Surgical or biopsy pathology was consistent with oncocytoma (47%), angiomyolipoma (43%), benign cysts (6%), oncocytic tumors (3%), and endometriosis (1%). Forty-nine (70%) patients received active treatment, while 21 patients (30%) elected surveillance. The most common complications included infection (7%) and blood loss requiring transfusion (3%).
The distribution of decisional regret is summarized in the following figure: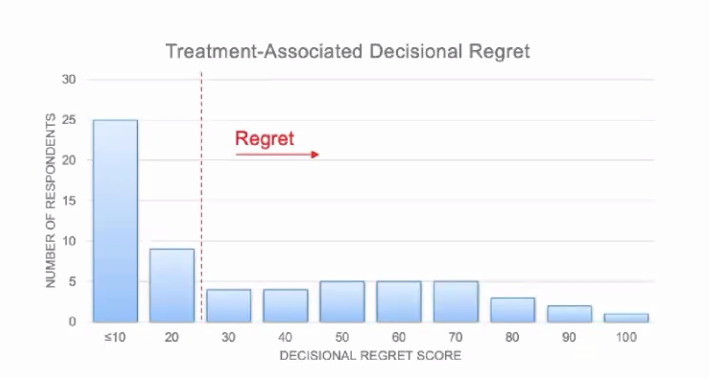
Decisional regret was expressed by 49% of patients and was associated with increasing age (p = 0.047), smaller tumor size (p = 0.036), and use of surveillance versus active treatment (p = 0.039) in univariate analysis. In multivariate analysis, only older age (p = 0.037) was significantly associated with decisional regret.
The distribution of financial toxicity is summarized in the following figure: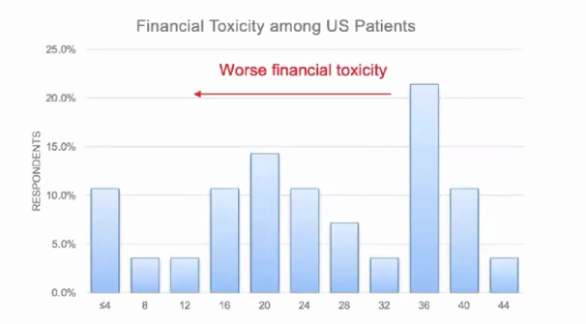
Younger age (p < 0.001) and diagnosis of angiomyolipoma (p = 0.047) were associated with greater financial toxicity in univariate linear regression, however, net income, treatment selection, and treatment associated complication were not associated with financial toxicity.
Dr. Mendhiratta concluded his presentation with the following summary points:
- High levels of treatment-associated decisional regret and financial toxicity were found among individuals with benign renal lesions
- Decisional regret and financial toxicity occurred independently of receiving active treatment, suggesting that overdiagnosis (not just over treatment) may be harmful to patients
- A diagnosis of a benign renal mass incurs a financial burden for patients in the United States
- Improved counseling and diagnostic tools may limit the psychological and financial burdens in select populations with these benign entities
- Further studies are needed to identify predictors of regret and financial toxicity and validate findings in a larger cohort
The second presentation was by Dr. Vikram M. Narayan, Assistant Professor at Emory University, discussing “Significant anti-adenovirus antibody response positively correlates with efficacy in patients treated with nadofaragene firadenovec for high-grade Bacillus Calmette Guérin (BCG) unresponsive nonmuscle-invasive bladder cancer (NMIBC).” Nadofaragene firadenovec is a non-replicating recombinant type-5 adenovirus vector-based gene therapy that delivers a copy of the human IFNα2b gene. Adenoviruses provide short-term, high but transient expression of the gene of interest in a relatively broad range of host cells. The Phase III trial of nadofaragene firadenovec for BCG unresponsive NMIBC was a multi-center study to investigate the safety and efficacy of intravesical nadofaragene firadenovec 75 mL once every 3 months in 157 patients with high-grade, BCG-unresponsive NMIBC. Cytology and cystoscopy (with biopsy if clinically indicated) were performed at 3, 6, and 9 months to evaluate for recurrence of high-grade disease. At 12 months, all patients underwent urine cytology, cystoscopy, and mandatory biopsy. Patients free from high-grade recurrence were eligible for retreatment at 3-month intervals while they remained high-grade recurrence-free. The study met its primary endpoint with 53.4% of patients with carcinoma in situ (CIS) ±Ta/T1 achieving a complete response, all by 3 months, including 43.6% of these patients remaining free of high-grade recurrence at 15 months. This study was recently published in Lancet Oncology.1
Non-replicating adenovirus vectors are widely used due to their advantages as a gene delivery vehicle. However, a crucial concern is that adenovirus vector-mediated transduction is suppressed in individuals with pre-existing anti-adenovirus neutralizing antibodies.
The most commonly used recombinant adenoviruses are based on adenovirus type 5 (Ad5). Most adults have pre-existing antibodies to adenovirus as a result of previous environmental exposure.
This analysis focused on the correlation of the anti-adenoviral antibody levels to response rate, a secondary objective of the trial. Blood samples for anti-adenoviral antibody level assessments were collected between 24 and 1-hour pre-dose on day 1 and 3, 6, 9, and 12 months or at a withdrawal-from-treatment study visit. A patient was considered to have a positive immunogenic response if a post-baseline anti-adenoviral antibodies titration demonstrated a > 2-fold dilution increase from baseline. This analysis was based on the data cut-off at 15 months.
Of the 151 patients included in the efficacy analysis, 129 had anti-adenoviral antibody titer results and were included in this analysis. Among the 55 patients who achieved a complete response in the CIS ± Ta/T1 cohort, significantly more patients had a positive post-baseline immunogenic response (43 vs. 8; p= 0.0033). This was similarly observed in the high-grade Ta/T1 cohort where among the 34 patients who remained free of high-grade recurrence at 3 months, significantly more patients had a positive post-baseline immunogenic response (30 vs. 4; p= 0.0003):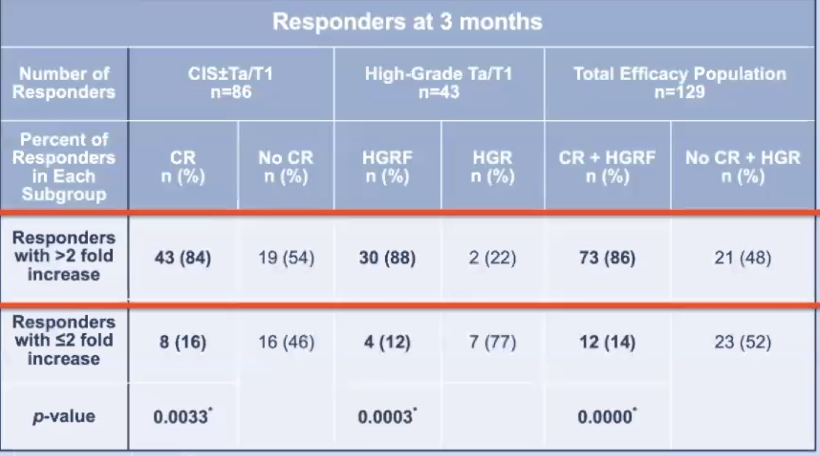
At 15 months of follow-up, the same trends were noted among patients who remained free of high-grade recurrence, with 19 vs. 3 (p=0.1032) in the CIS ± Ta/T1 cohort and 17 vs. 2 (p=0.08) patients in the high-grade Ta/T1 cohort who had a post-baseline immunogenic response.
Dr. Narayan concluded his presentation with the following take-home messages:
- Nadofaragene firadenovec instilled intravesically once every 3 months achieves durable complete responses in patients with high-grade, BCG-unresponsive NMIBC
- Titer data suggest that a significant anti-adenovirus antibody response is associated with treatment response and may be used to identify responders
- Community exposure to adenoviruses does not appear to preclude efficacy of the agent
- Nadofaragene firadenovec demonstrates promising efficacy and is a potential novel therapeutic option in a patient population with an urgent unmet medical need
Neil Mendhiratta, MD, University of California Los Angeles (UCLA), Los Angeles, California
Vikram M. Narayan, MD, Director of Urological Oncology, Grady Memorial Hospital, Assistant Professor of Urology, Department of Urology, Emory University School of Medicine, Emory University, Atlanta, Georgia
Written by: Zachary Klaassen, MD, MSc, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Augusta, Georgia, Twitter: @zklaassen_md at the 2020 Society of Urologic Oncology Annual Meeting – December 2-5, 2020 – Washington, DC
Reference:
1. Boorjian, Stephen A., Mehrdad Alemozaffar, Badrinath R. Konety, Neal D. Shore, Leonard G. Gomella, Ashish M. Kamat, Trinity J. Bivalacqua et al. "Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial." The Lancet Oncology (2020).


