Cytology and cystoscopy (with biopsy if clinically indicated) were performed at 3, 6, and 9 months to evaluate for recurrence of high-grade disease. At 12 months, all patients underwent urine cytology, cystoscopy, and mandatory biopsy. Patients free from high-grade recurrence were eligible for retreatment at 3-month intervals while they remained high-grade recurrence-free. The study met its primary endpoint with 53.4% of patients with CIS ±Ta/T1 achieving a complete response, all by 3 months, including 43.6% of these patients remaining free of high-grade recurrence at 15 months. This study was recently published in Lancet Oncology.1
At the 2020 SUO virtual meeting, Dr. Vikram M. Narayan, Assistant Professor at Emory University, discussed the results of their additional analysis assessing anti-adenovirus antibody response correlating with efficacy among patients in this study.
Non-replicating adenovirus vectors are widely used due to their advantages as a gene delivery vehicle. However, a crucial concern is that adenovirus vector-mediated transduction is suppressed in individuals with pre-existing anti-adenovirus neutralizing antibodies.
The most commonly used recombinant adenovirus is based on adenovirus type 5 (Ad5). Most adults have pre-existing antibodies to adenovirus as a result of previous environmental exposure.
This analysis focused on correlation of the anti-adenoviral antibody levels to response rate, a secondary objective of the trial.
For this study, blood samples for anti-adenoviral antibody level assessments were collected between 24 and 1-hour pre-dose on day 1 and 3, 6, 9, and 12 months or at a withdrawal-from-treatment study visit. A patient was considered to have a positive immunogenic response if a post-baseline anti-adenoviral antibodies titration demonstrated a >2-fold dilution increase from baseline. This analysis was based on the data cut-off at 15 months.
Of the 151 patients included in the efficacy analysis, 129 had anti-adenoviral antibody titer results and were included in this analysis. Among the 55 patients who achieved complete response in the CIS ± Ta/T1 cohort, significantly more patients had positive post-baseline immunogenic response (43 vs 8; p= 0.0033). This was similarly observed in the high-grade Ta/T1 cohort where among the 34 patients who remained free of high-grade recurrence at 3 months, significantly more patients had positive post-baseline immunogenic response (30 vs 4; p= 0.0003):
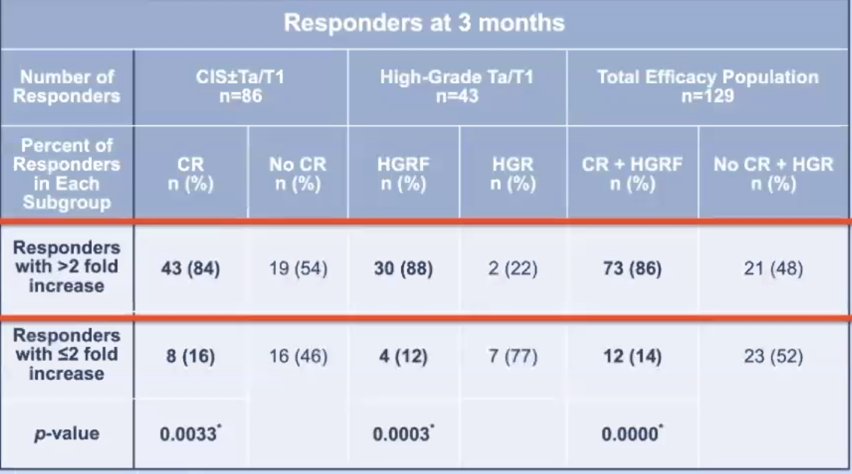
At 15 months of follow-up, the same trends were noted among patients who remained free of high-grade recurrence, with 19 vs 3 (p=0.1032) in the CIS ± Ta/T1 cohort and 17 vs 2 (p=0.08) patients in the high-grade Ta/T1 cohort who had post-baseline immunogenic response.
Dr. Narayan concluded his presentation with the following take-home messages:
- Nadofaragene firadenovec instilled intravesically once every 3 months achieves durable complete responses in patients with high-grade, BCG-unresponsive NMIBC
- Titer data suggest that significant anti-adenovirus antibody response is associated with treatment response and may be used to identify responders
- Community exposure to adenoviruses does not appear to preclude efficacy of the agent
- Nadofaragene firadenovec demonstrates promising efficacy and is a potential novel therapeutic option in a patient population with an urgent unmet medical need
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2020 Society of Urologic Oncology Annual Meeting – December 2-5, 2020 – Washington, DC
References:
Related Content:
SUO 2020: Subgroup Analyses of the Phase 3 Study of Intravesical Nadofaragene Firadenovec in Patients with High-Grade, Bacillus Calmette Guerin (BCG)-Unresponsive Non Muscle Invasive Bladder Cancer (NMIBC)


