The PROSPER trial is a multinational, double-blinded, randomized, placebo-controlled phase 3 trial that was developed to evaluate the efficacy of enzalutamide in the treatment of nonmetastatic, castration-resistant prostate cancer (nmCRPC). Enzalutamide is an antiandrogen that is used in combination with androgen deprivation therapy (ADT) and was first approved for the treatment of nmCRPC in 2018 after the initial results of the PROSPER trial were published.1
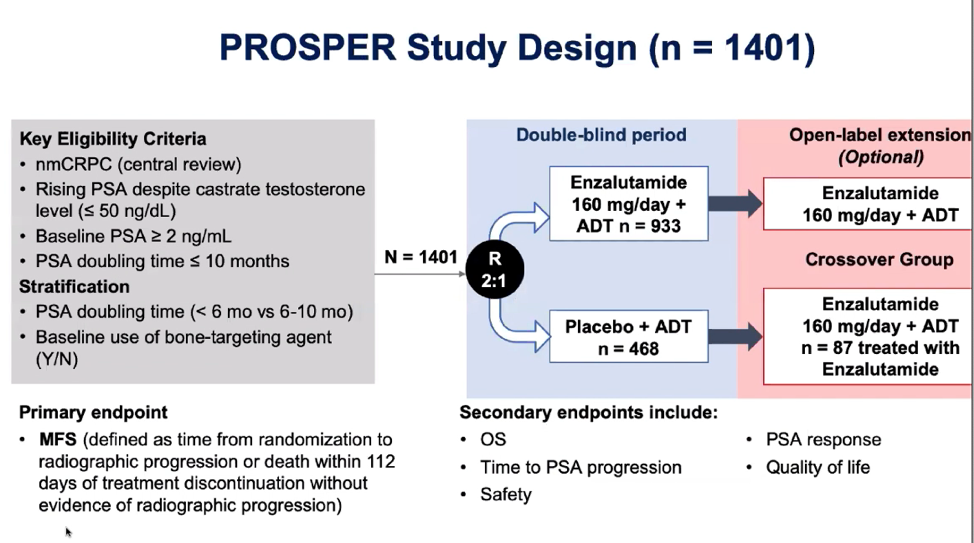
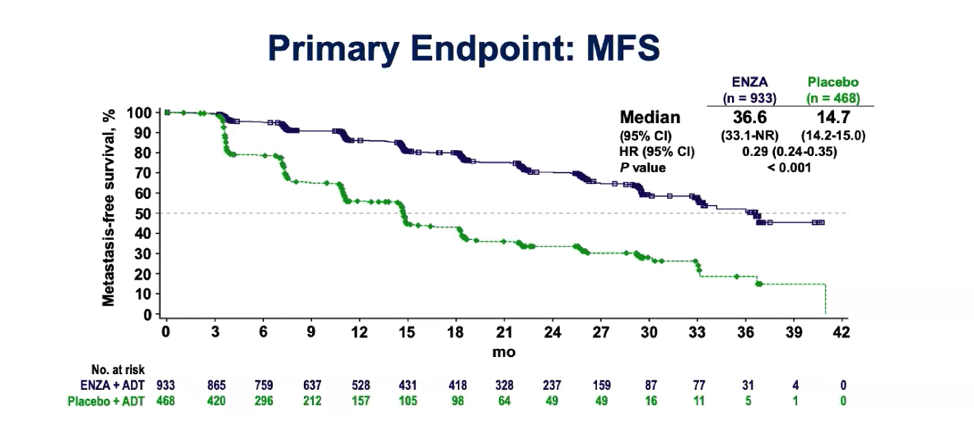
Historically, patients with nmCRPC progress rapidly to bone metastases, which is then quickly followed by further disease progression and death. The primary endpoint of this trial was metastasis-free survival (MFS) and the authors demonstrated a 71% lower risk of radiographic progression or death in the enzalutamide arm as compared to ADT alone (Figure 2). Furthermore, patients in the enzalutamide arm experienced a delay to subsequent antineoplastic therapy.
Next, Dr. Sternberg presented the most recent interim results of the PROSPER trial which were published this summer in the New England Journal of Medicine.2 Overall, they demonstrated a 27% reduction in risk of overall death at 48-month follow-up (Figure 3). This is impressive given the fact that 84% of the placebo group had received additional antineoplastic agents once unblinded, including 36% which received enzalutamide later in their disease course. Additionally, the trial demonstrated a favorable safety profile, which is key as patients remained on treatment for a median duration of 34 months.
During the same period of time, both apalutamide and darolutamide have also been tested within this same patient population in the SPARTAN and ARAMIS trials respectively. The three trials were designed similarly with the primary endpoint of MFS and the secondary endpoint overall survival, with all demonstrating a similar reduction in risk of death. Median overall survival, however, has not yet been reached in the ARAMIS trial. Despite these similarities, Dr. Sternberg cautioned that the medications have not been studied in head to head trials and that direct comparisons between the groups should be limited.
Dr. Sternberg concluded her talk by reiterating that in the setting of nmCRPC, earlier treatment appears to prolong patient survival and this has been reflected by changes to the AUA and NCCN guidelines for all three antiandrogens.
Presented by: Cora N. Sternberg, MD, Weill Cornell Medicine, New York, New York
Written by: Adrien Bernstein, MD, Society of Urologic Oncology Fellow, Fox Chase Cancer Center, Fox Chase Cancer Center, Philadelphia, PA, at the 21th Annual Meeting of the Society of Urologic Oncology (SUO), December 3-5, Virtual Conference
References:
- Hussain, M. et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. New Engl J Med 378, 2465–2474 (2018).
- Sternberg, C. N. et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. New Engl J Med 382, 2197–2206 (2020).
Related Content:
The PROSPER Trial: Enzalutamide Demonstrates Significant Improvement in Overall Survival in Nonmetastatic Castration-Resistant Prostate Cancer - Cora Sternberg


