(UroToday.com) The Society of Urologic Oncology (SUO) annual winter meeting included a kidney cancer session and a presentation by Dr. Jose Karam discussing updates to the new AUA guidelines for the evaluation and management of clinically localized sporadic renal masses.1,2 Dr. Karam noted that the methodology for this guideline is comparable to previous AUA guidelines, including a rigorous, evidence-based approach, extensive peer review, systematic review and meta-analysis, and multiple conference calls. The follow-up for clinically localized renal neoplasms guideline was published in 2013 and was merged with the renal mass and localized renal cancer guideline from 2017. For this amendment, a literature search retrieved studies published between July 2016 and October 2020, identifying 19 new studies.
Dr. Karam started by giving a high-level overview of the 2021 updated guidelines, as follows:
- Merging of the two guidelines (2013 and 2017)
- Renal mass biopsy: indications more clearly defined
- Genetic counseling: indications expanded
- Radical nephrectomy versus partial nephrectomy: indications more granular and clinically useful
- Adjuvant therapy: addressed for the first time
- Active surveillance protocols: indications more granular
- Risk-based surveillance protocols after intervention: integrated and updated
For evaluation, an MRI with second generation gadolinium-based intravenous contrast is now a safer option in many patients with severe chronic kidney disease. A consensus statement from the American College of Radiology and the National Kidney Foundation suggests that such agents can be given to patients with a GFR under 30 ml/min/1.73m2. Furthermore, the risks of nephrogenic systemic fibrosis using second generation gadolinium-based agents is less than 0.07%. In a concurrent American College of Radiology guideline, they state that patients need not be screened for renal function prior to receiving second generation gadolinium-based agents.
For renal mass biopsy, Dr. Karam notes that in the setting of a solid renal mass a biopsy should be obtained on a utility-based approach whenever it may influence management. A renal mass biopsy is not required for (i) young or healthy patients who are unwilling to accept the uncertainties associated with a biopsy, or (ii) older or frail patients who will be managed conservatively independent of renal mass biopsy findings. Renal mass biopsy should be performed prior to (preferred) or at the time of ablation to provide pathologic diagnosis and guide subsequent surveillance.
For genetic counseling, clinicians should recommend testing for any of the following:
- Patient is <= 46 years of age with renal malignancy
- Multifocal or bilateral renal masses
- Personal history suggests a familial renal neoplastic syndrome
- First or second-degree relative with a history of renal malignancy or a known clinical or genetic diagnosis of a familial renal neoplastic syndrome (even if kidney cancer has not been observed)
- Pathology demonstrates histologic findings suggestive of such a syndrome
With regards to indications for radical nephrectomy versus partial nephrectomy, clinicians should consider a radical nephrectomy for patients with a solid or Bosniak 3-4 complex cystic renal mass whenever increased oncologic potential is suggested by tumor size, renal mass biopsy (if obtained), and/or imaging. More specifically, radical nephrectomy is preferred if all of the following criteria are met:
- High tumor complexity and partial nephrectomy would be challenging even in experienced hands
- No pre-existing chronic kidney disease or proteinuria
- Normal contralateral kidney and new baseline eGFR will likely be greater than 45 mL/min/1.73m2
If all of these criteria are not met, partial nephrectomy should be considered unless there are overriding concerns about the safety or oncologic efficacy of partial nephrectomy.
Clinicians should consider a referral to medical oncology whenever there is concern for potential clinical metastasis or incompletely resected disease (macroscopic positive margin or gross residual disease). Additionally, patients with high-risk or locally advanced, fully resected renal cancers should be counselled about the risks/benefits of adjuvant therapy and encouraged to participate in adjuvant clinical trials, facilitated by medical oncology consultation when needed.
For patients with a solid or Bosniak 3/4 complex cystic renal mass in whom the risk/benefit analysis for treatment is equivocal and who prefer active surveillance, clinicians should consider renal mass biopsy (if the mass is solid or has solid components) for further oncologic risk stratification. Repeat cross-sectional imaging should be obtained approximately 3-6 months later to assess for interval growth. Periodic clinical/imaging surveillance can then be based on growth rate and shared decision-making with intervention recommended if substantial interval growth is observed or if other clinical/imaging findings suggest that the risk/benefit analysis is no longer equivocal or favorable for continued active surveillance. Furthermore, for patients with a solid or Bosniak 3/4 complex cystic renal mass in whom the anticipated oncologic benefits of intervention outweigh the risks of treatment and competing risks of death, clinicians should recommend intervention. Active surveillance with potential for delayed intervention may be pursued only if the patient understands and is willing to accept the associated oncologic risks. In this setting, clinicians should encourage renal mass biopsy (if the mass is predominantly solid) for additional risk stratification. If the patient continues to prefer active surveillance, close clinical and cross-sectional imaging surveillance with periodic reassessment and counseling should be recommended.
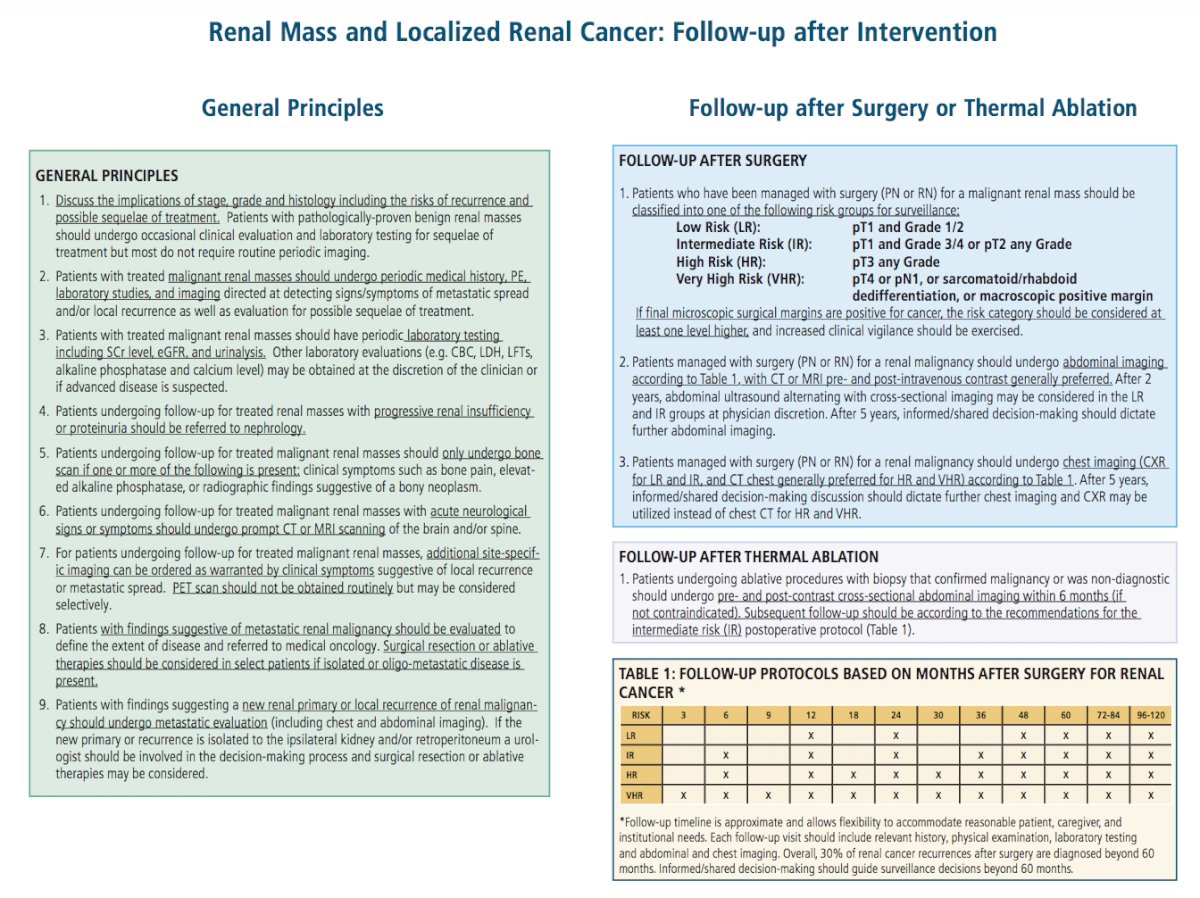
Clinicians should classify patients who have been managed with surgery (partial nephrectomy or radical nephrectomy) for a malignant renal mass into one of the following risk groups:
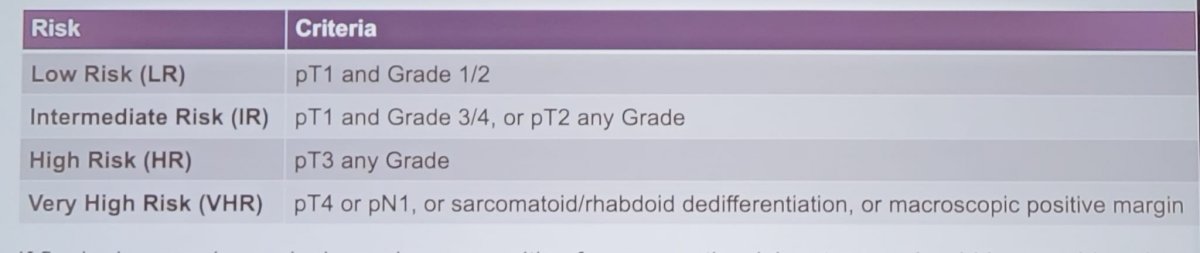
The recommended follow-up schedule after surgery for kidney cancer is as follows:
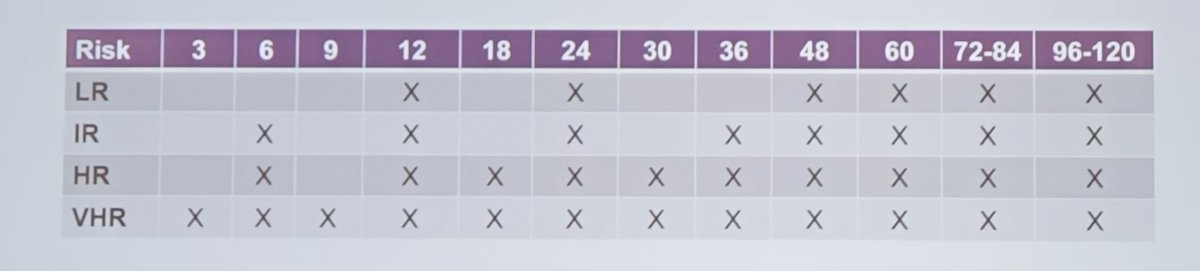
This follow-up timeline is approximate and allows flexibility to accommodate reasonable patient, caregiver, and institutional needs. Each follow-up visit should include relevant history, physical examination, laboratory testing, and abdominal and chest imaging. Informed/shared decision making should guide surveillance decisions beyond 60 months.
Patients undergoing ablative procedures with biopsy that confirmed malignancy or was non-diagnostic should undergo pre- and post-contrast cross sectional abdominal imaging within 6 months (if not contraindicated). Additionally, subsequent follow-up should be according to the recommendations for the intermediate risk post-operative protocol.
Dr. Karam concluded by highlighting the updated figures that accompany this 2021 guideline:
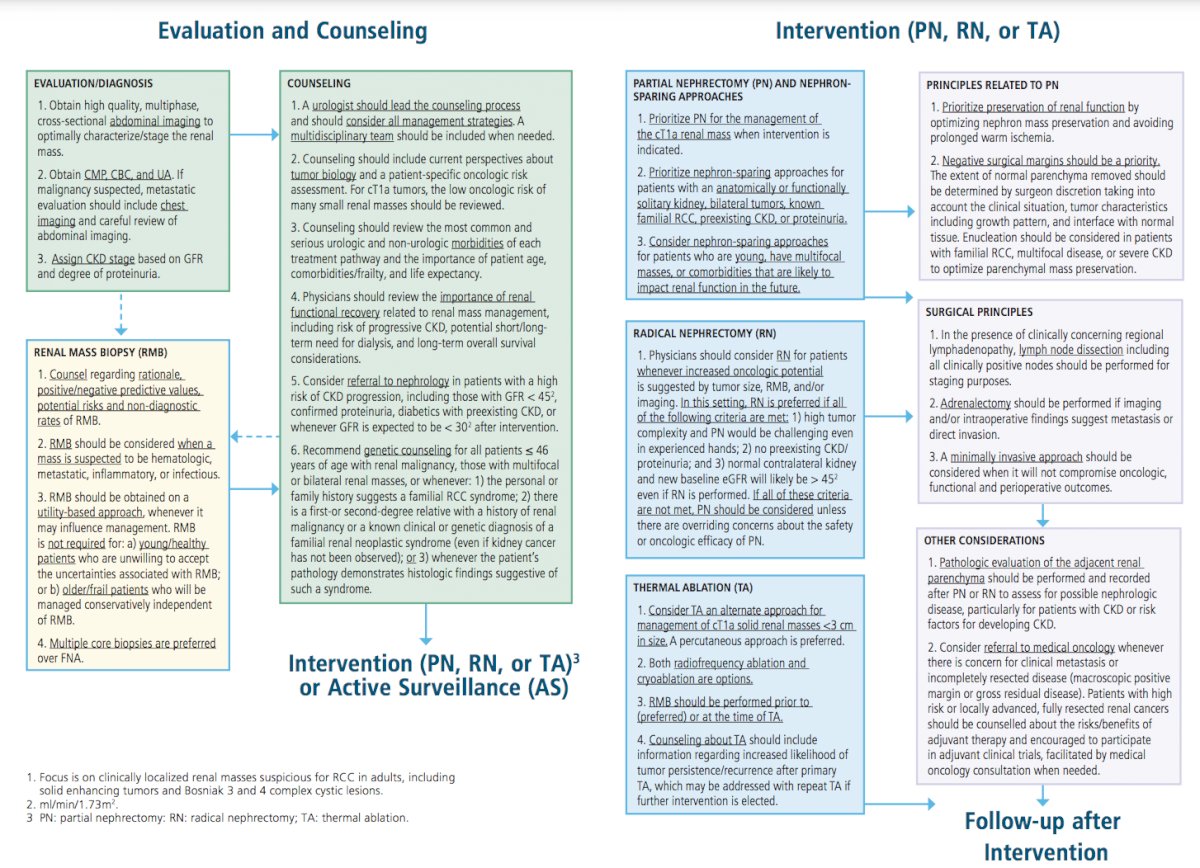
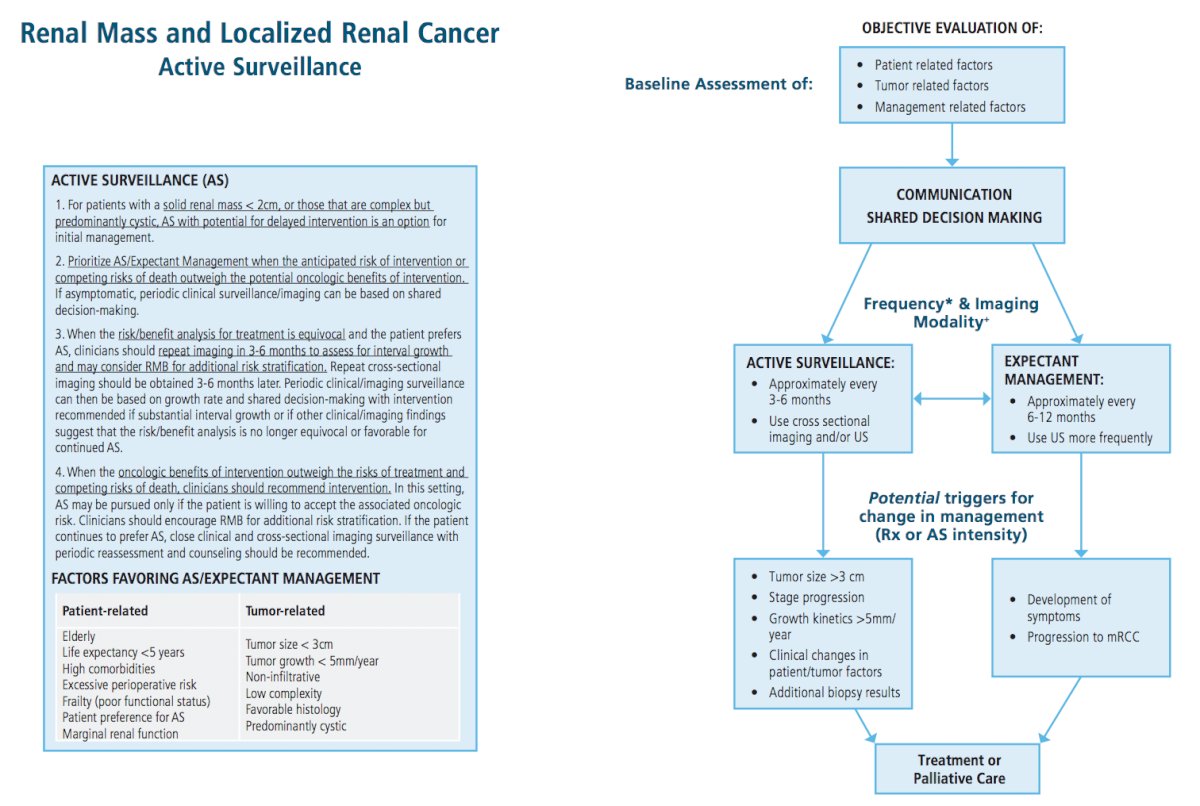
Presented by: Jose A. Karam, M.D. Anderson Cancer Center, Houston, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 Society of Urologic Oncology (SUO) Winter Annual Meeting, Orlando, FL, Wed, Dec 1 – Fri, Dec 3, 2021.
References:
- Campbell SC, Clark PE, Chang SS, et al. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-Up: AUA Guideline: Part I. J Urol. 2021 Aug;206(2):199-208.
- Campbell SC, Uzzo RG, Karam JA, et al. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-Up: AUA Guideline: Part II. J Urol. 2021 Aug;206(2):209-218.


