(UroToday.com) The Society of Urologic Oncology (SUO) annual winter meeting included a session on the risk stratification of prostate cancer and a presentation by medical oncologist Dr. Alicia Morgans discussing the incorporation of molecular markers and genomic studies into advanced prostate cancer therapy. Dr. Morgans notes that according to the NCCN Guidelines Version 2.2022 for prostate cancer, germline testing is specifically recommended for patients with metastatic, regional (node positive), very-high risk localized, and high-risk localized prostate cancer. Additionally, germline testing may be considered in patients with a personal history of prostate cancer and those with:
- Intermediate-risk prostate cancer with intraductal/cribriform histology
- A personal history of any of the following cancers: exocrine pancreatic, colorectal, gastric, melanoma, pancreatic, upper tract urothelial, glioblastoma, biliary tract, and small intestinal
With regards to somatic tumor testing, Dr. Morgans notes that the NCCN guidelines also makes the following recommendations:
- Tumor testing for alterations in homologous recombination DNA repair genes, such as BRCA1, BRCA2, ATM, PALB2, FANCA, RAD51D, CHEK2, and CDK12, is recommended in patients with metastatic prostate cancer. This testing can be considered in patients with regional prostate cancer
- Tumor testing for microsatellite instability high (MSI-H) or deficient mismatch repair (dMMR) is recommended in patients with metastatic castration-resistant prostate cancer (mCRPC) and may be considered in patients with regional or castration-native metastatic prostate cancer
- Tumor mutational burden testing may be considered in patients with mCRPC
Dr. Morgans emphasized that the AUA/ASTRO/SUO guideline on advanced prostate cancer also makes several recommendations with regards to genetic testing: (i) in patients with metastatic hormone sensitive prostate cancer, regardless of age and family history, clinicians should offer genetic counseling and germline testing (Expert Opinion), (ii) in patients with mCRPC, clinicians should offer germline and somatic tumor genetic testing to identify DNA repair deficiency mutations and microsatellite instability status that may inform prognosis and counseling regarding family risks, as well as potential targeted therapies (Expert Opinion).
Indeed, DNA repair gene alterations are common in metastatic prostate cancer, with ~23% of patients with mCRPC harboring a DNA repair alteration and the frequency of DNA repair alterations increasing with disease progression. In the seminal work from Pritchard and colleagues1 among men with metastatic prostate cancer, 11.8% had a germline mutation in 16 DNA damage repair genes. Age and family history in this study did not affect mutation frequency.
The PROfound study was a phase III trial of men with mCRPC who had progressed on previous abiraterone acetate or enzalutamide.2 The investigators used the FoundationOne CDx assay to identify alterations in one of 15 pre-specified genes involved in homologous recombination repair (BRCA 1/2, ATM, BRIP1, BARD1, CDK12, CHEK 1/2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, RAD54L). Cohort A included patients with alterations in BRCA1, BRCA2, or ATM while Cohort B had alterations in any of the other 12 included genes. In both cohorts, patients were randomized 2:1 to olaparib vs. abiraterone or enzalutamide. The primary analysis was based on imaging-based progression free survival (soft-tissue according to RESIST 1.1 and bony according to PCCTWG3 criteria) among patients in Cohort A:
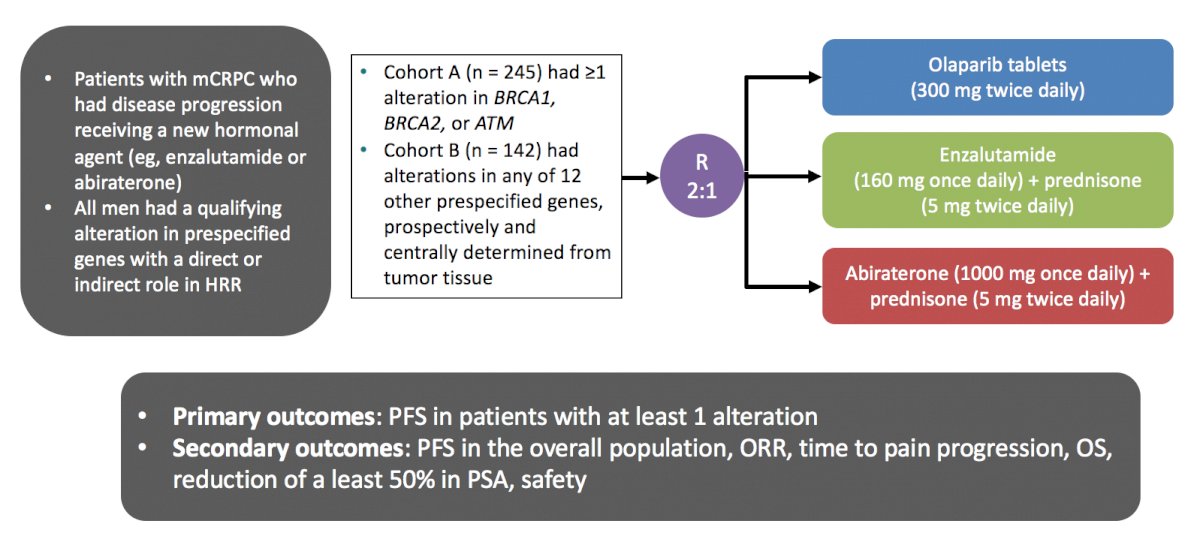
There was a significantly improved progression-free survival in patients with mutations of BRCA1, BRCA2, or ATM (HR 0.34, 95% CI 0.25 to 0.47), and similar results were seen in the combined cohort (HR 0.49, 95% CI 0.38 to 0.63). An updated analysis also showed a survival benefit for olaparib in the overall cohort with a median OS of 17.3 months compared to 14.0 months in the control arm (HR 0.79, 95% CI 0.61-1.03).3 Furthermore, there was a significant survival benefit for olaparib with a median OS of 19.1 months compared to 14.7 months in the control arm in Cohort A (HR 0.69, 95% CI 0.50-0.97):
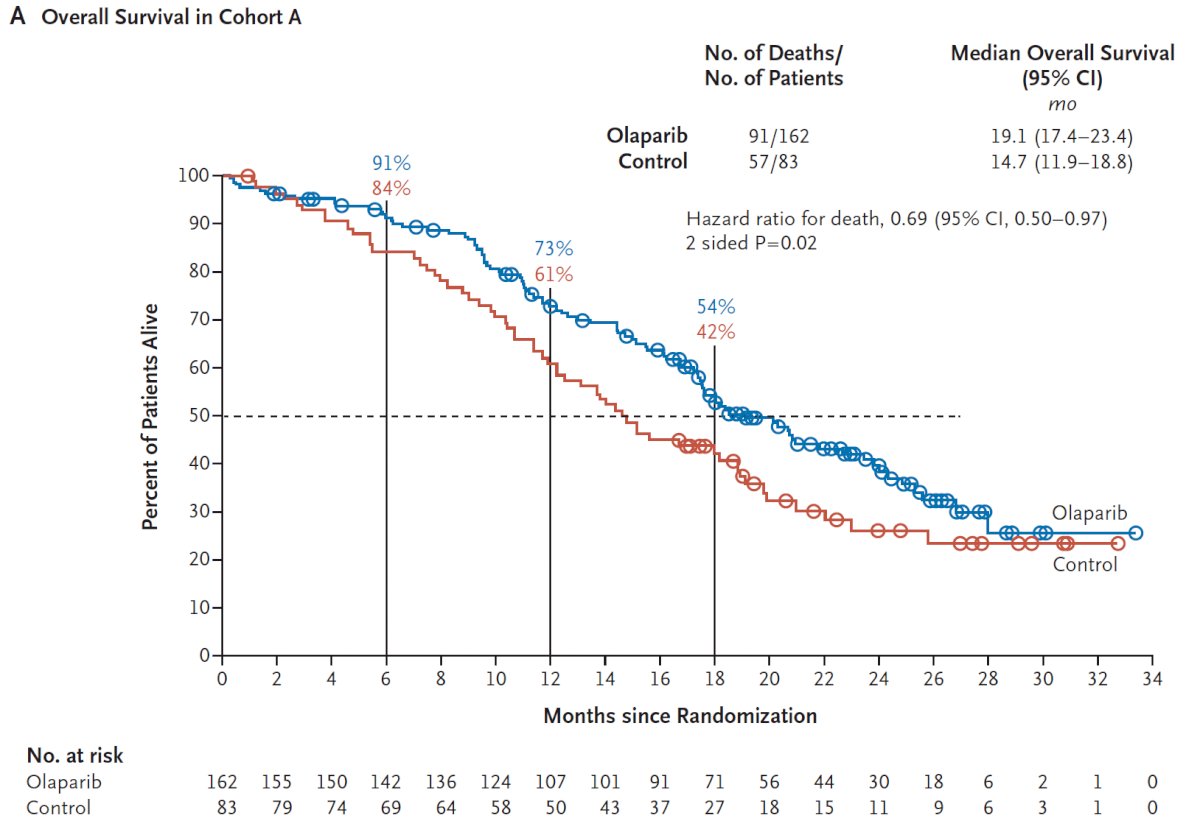
Adverse events were common in both patients on olaparib (any = 95%, grade ≥ 3 = 51%) and in the control group (any = 88%, grade ≥ 3 = 38%).
The TRITON2 trial4 assessed rucaparib 600 mg BID in patients with mCRPC associated homologous recombination repair gene alterations. The study design for this trial is as follows:
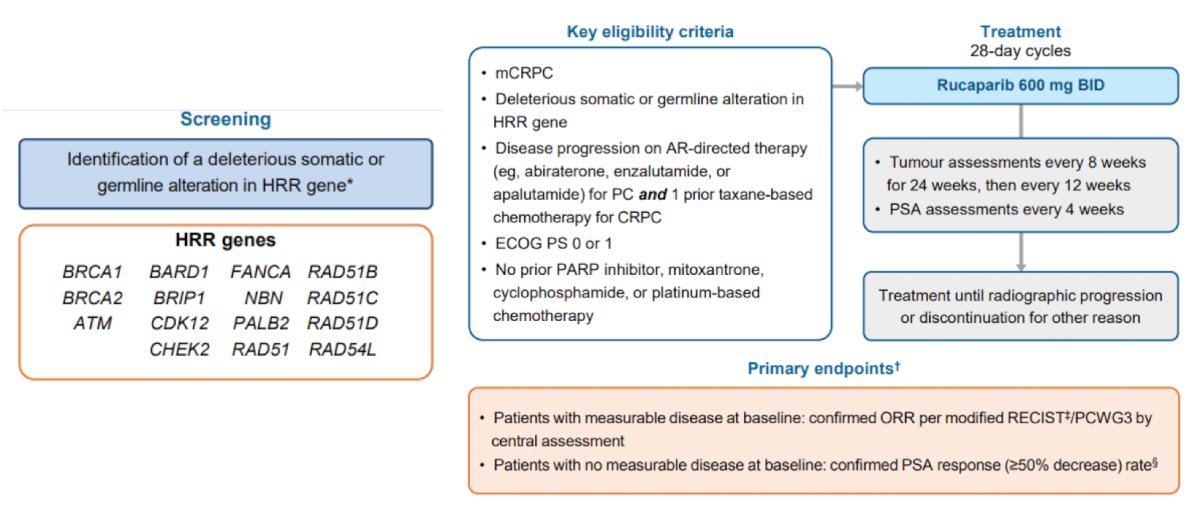
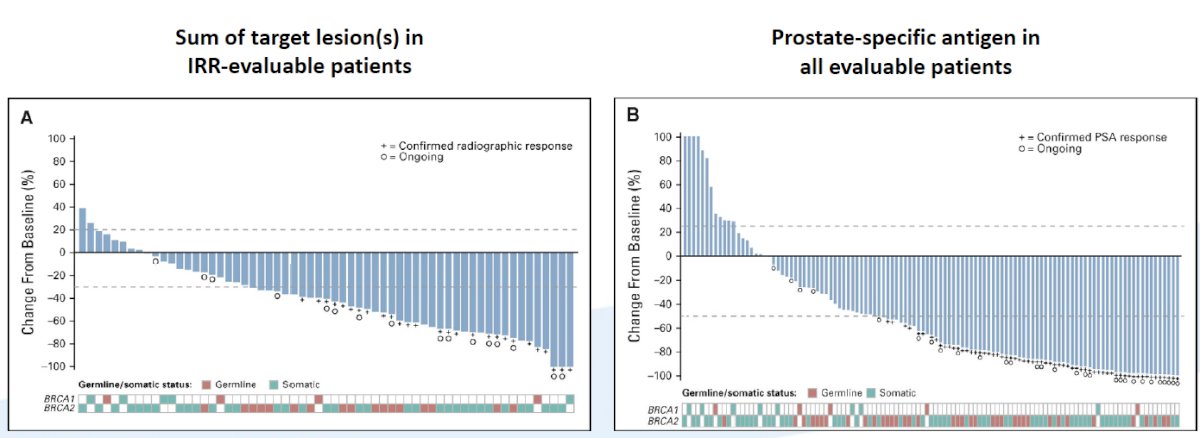
For the patients with BRCA1/2 alteration, there was a 43.5% confirmed overall response rate and 54.8% confirmed PSA response rate while patients harboring an ATM and CDK12 alteration did not receive significant benefit. As follows is the best change from baseline for the sum of the target lesions and PSA in evaluable patients:
Patients receiving rucaparib in TRITON2 generally tolerated treatment well, with grade >=3 adverse events notable for anemia (25.2%), thrombocytopenia (9.6%), and asthenia/fatigue (8.7%).
Based on the aforementioned clinical trials assessing PARP inhibitors in mCRPC patients, the FDA provided accelerated approval on May 15, 2020, for rucaparib for the treatment of patients with deleterious BRCA1/2 (germline and/or somatic)-associated mCRPC who have been treated with an androgen receptor-directed therapy and taxane-based chemotherapy. With regards to olaparib, on May 19, 2020, the FDA approved olaparib for the treatment of patients with pathogenic germline or somatic HRR gene-mutated mCRPC who have progressed following prior treatment with enzalutamide or abiraterone.
Dr. Morgans notes that pembrolizumab is also a potential option for mCRPC patients, receiving FDA approval in a tumor agnostic indication for MSI-high (MSI-H) mutation CRPC patients in 2017. Approximately 2-3% of men with prostate cancer have MSI-H tumors and can have radiographic responses to pembrolizumab.
With regards to approaches to molecular testing, the NCCN guidelines strongly recommend a metastatic biopsy for histologic and molecular evaluation. When unsafe or unfeasible, plasma ctDNA assay is an option, however, caution is needed when interpreting ctDNA-only evaluation due to the potential for interference from clonal hematopoiesis of indeterminate potential (CHIP), which can result in false-positive biomarker signal. Strengths and weaknesses of testing approaches are summarized in the following figure:
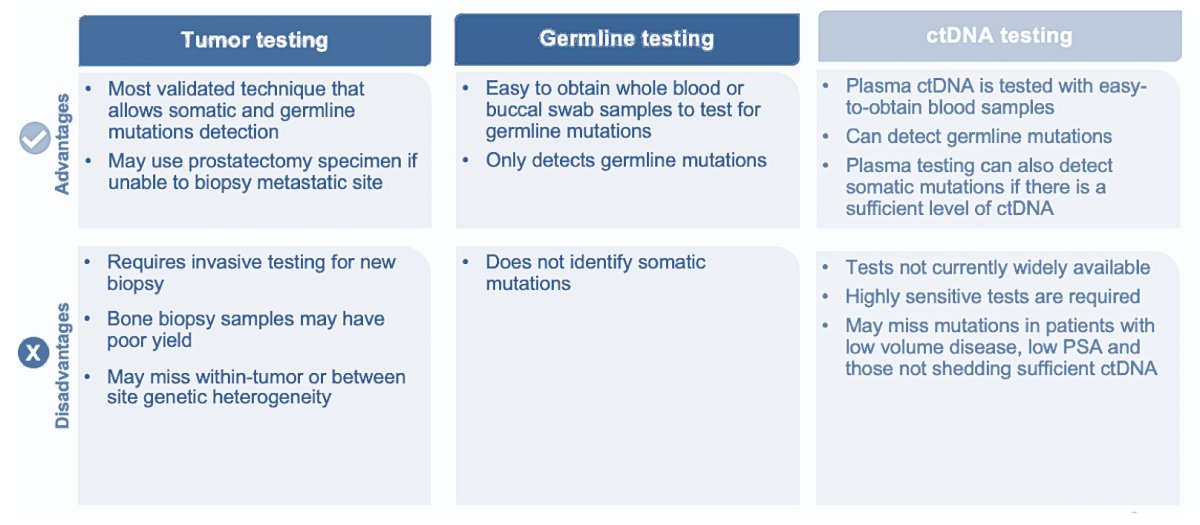
Dr. Morgans notes that there are several factors that may affect the success of cell-free DNA for somatic testing, noting that there is a higher likelihood for success in patients with a high PSA, high-volume disease, and/or castration-resistant prostate cancer. In fact, somatic mutations are identified in cell-free DNA in 93% of men with a PSA > 10 ng/mL. If DNA analysis for MSI is required, the NCCN guidelines note that using a next-generation sequencing assay validated for prostate cancer is the preferred method.
Future directions in this disease space are likely to continue to explore targeted and combination therapies, as highlighted in the following table of ongoing PARP inhibitor trials assessing combinations with immune checkpoint inhibitors, chemotherapy, and radionuclide therapies:
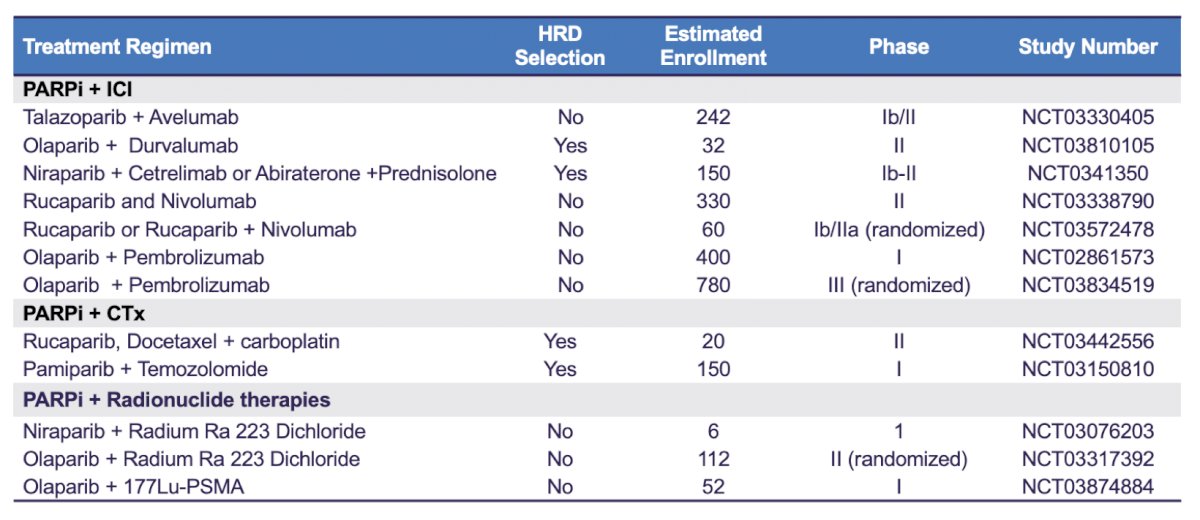
Additionally, there are ongoing androgen receptor-targeted combination trials in order to improve the “BRCA-ness” of tumors and perhaps response to PARP inhibitors, including:
- PROpel (NCT03732820): A phase 3 study of abiraterone +/- olaparib in mCRPC
- MAGNITUDE (NCT03748641): A phase 3 study of abiraterone +/- niraparib in mCRPC
- CASPAR (NCT04455750): A phase 3 study of enzalutamide +/- rucaparib in mCRPC
- AMPLITUDE (NCT04497844): A phase 3 study of abiraterone +/- niraparib in HRR in mHSPC
Of note, in a press release on September 24, 2021, it was announced that “Lynparza in combination with abiraterone significantly delayed disease progression in all-comers in the PROpel Phase III trial in 1st-line metastatic castration-resistant prostate cancer.”
Dr. Morgans concluded her presentation of incorporating molecular markers and genomic studies into therapy for advanced prostate cancer with the following take-home messages:
- PARP inhibitors (olaparib and rucaparib) are approved for the treatment of mCRPC patients with DDR mutations, however not all DDR mutations respond the same
- Pembrolizumab is approved for the treatment of patients with MSI-H tumors
- Guidelines recommend germline and tumor testing to identify targetable alterations. Awareness of factors associated with success (higher PSA, higher disease volume) and CHIP are critical when using liquid biopsy approaches
- Future targeted treatments and combinations are on the horizon
Presented by: Alicia Morgans, MD, MPH, Medical Oncologist, Dana Farber Cancer Institute, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 Society of Urologic Oncology (SUO) Winter Annual Meeting, Orlando, FL, Wed, Dec 1 – Fri, Dec 3, 2021.
References:
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020 May 28;382(22):2091-2102.
- Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020 Dec 10;383(24):2345-2357.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol 2020 Nov 10;38(32):3763-3772.


