(UroToday.com) The Society of Urologic Oncology (SUO) 2021 annual meeting in Orlando, FL included a comprehensive overview by Dr. Thomas Hope of “Integrating Imaging into Prostate Cancer Care”, specifically with regards to the diagnostic role of Prostate Specific Membrane Antigen (PSMA)-based positron emission tomography (PET) imaging in the different phases of prostate cancer management.
Dr. Hope started his presentation with a case study of a 69-year-old man with a prostate-specific antigen (PSA) level of 0.67 post-radical prostatectomy and demonstrated anecdotally the value of Ga-based PSMA imaging in the detection of two distinct disease spread sites- regionally in the left internal iliac node and distally in the right humerus. Dr. Hope went on to highlight the December 2020 Food and Drug Association’s (FDA) approval of 68-PSMA-11 based PET imaging in prostate cancer men with (i) suspected metastasis who are candidates for initial definitive therapy or (ii) suspected recurrence based on elevated serum PSA levels.

Dr. Hope next presented a comparative analysis by Calais et al. who demonstrated the overall diagnostic superiority of 68Ga-PSMA-11 imaging compared to Fluciclovine PET/CT, with an overall diagnostic accuracy of 56% compared to 26%, in favor of PSMA. This superiority was most evident in the diagnosis of nodal disease (30% versus 8% in favor of PSMA) and any metastatic disease (16% versus 0% in favor of PSMA). Notably, the inter-reader variability (k) was vastly superior with PSMA imaging (0.67 versus 0.20).1
In addition to 68Ga-PSMA-11, currently available molecules include 18F-DCFPyL (PYLARIFY®), PSMA-617, PSMA-1007, and rh-PSMA-7. These molecules vary in their routine uptake and subsequent biodistribution, which is crucial to be aware of when interpreting these imaging modalities.
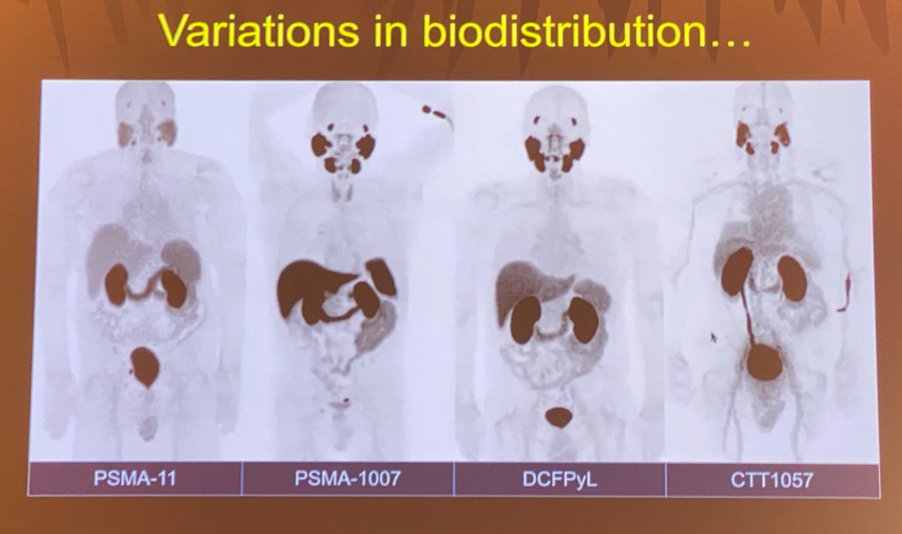
Next, Dr. Hope provided a brief overview of the two trials evaluating 18F-DCFPyL PET imaging: the OSPREY2 and CONDOR3 trials.
The OSPREY trial included two patient cohorts:
- Cohort A: Initial staging cohort (n=252) with a specificity of 98% and sensitivity of 40%
- Cohort B: Biochemical recurrence cohort (n=93), with a median PSA of 11.3 ng/ml. The sensitivity of DCFPyL PET imaging in this group was 96%, with a positive predictive value of 82%.
Conversely, the CONDOR trial included only patients with biochemical recurrence (n=208) and did not require biopsiable lesions. Notably, the median PSA level in this cohort was 0.8 ng/ml (compared to 11.3 ng/ml in OSPREY). The correct localization rate was 85-87%, with a detection rate of 59-66%.
Dr. Hope next went on to highlight the recent National Comprehensive Cancer Network (NCCN) changes from September 10, 2021, that included the following statement: “Because of the increased sensitivity and specificity of PSMA-PET tracers for detecting micrometastatic disease compared to conventional imaging (CT, MRI) at both initial staging and biochemical recurrence, the Panel does not feel that conventional imaging is a necessary prerequisite to PSMA-PET and that PSMA-PET/CT or PSMA-PET/MRI can serve as an equally effective, if not more effective front-line imaging tool for these patients”. The NCCN panel elaborated further by clearly stating that these imaging modalities may be considered in the following disease spaces:
- Initial imaging (unfavorable intermediate, high, and very high-risk patients)
- Biochemical recurrence (no PSA cutoff)
- Progression for castration sensitive prostate cancer patients on systemic therapy
- Progression with M0 castration resistant prostate cancer
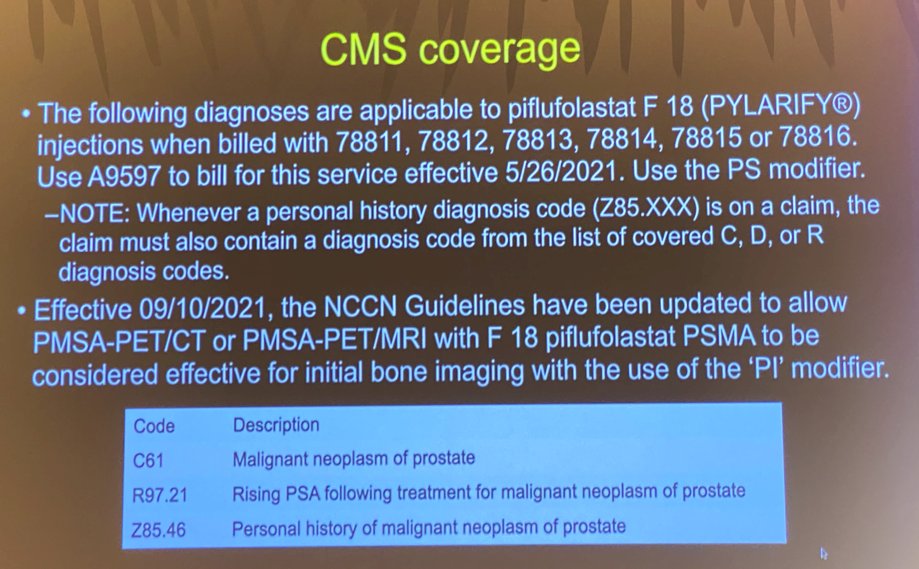
Consequently, Centers for Medicare & Medicaid Services (CMS) coverage for these imaging modalities has been adopted as highlighted in the figure below.
Despite the increased sensitivity/accuracy of these imaging modalities, caution with regards to potential false-positive interpretations remains important.
Notable benign false-positive lesions include:
- Rib lesions
- Pre-sacral ganglia
- Dorsal root ganglia
- Hemangiomas
- Paget’s disease
Other PSMA-avid tumors include:
- Hepatocellular carcinoma
- Thyroid cancer
- Lung cancer
Another common interpretive pitfall occurs when the tracer is excreted by the kidney into the ureters and nodes adjacent to the avid tracer are erroneously labeled as being positive for presence of tumor.
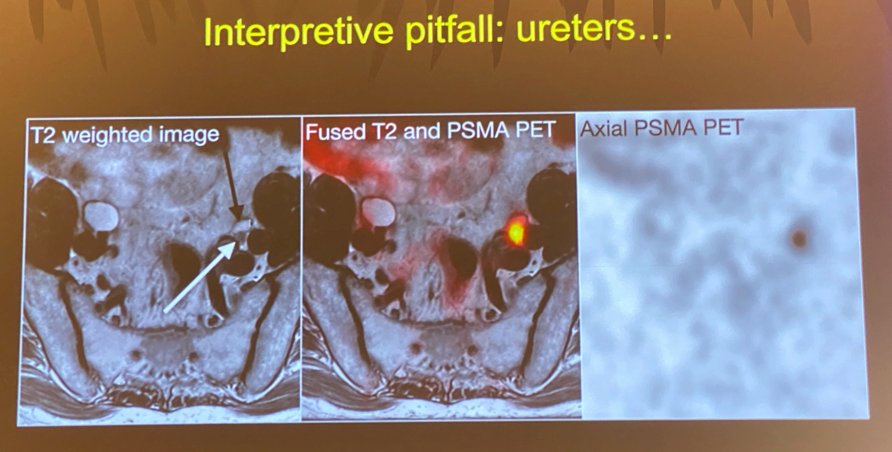
Similarly, when the tracer accumulates in the bladder, this may be falsely interpreted as “local recurrence” in the prostate bed.

Dr. Hope emphasized that despite the obvious advantages of PSMA imaging, there remains a small proportion of PSMA negative tumors with restricted uptake of tracer in the prostate itself. Dr. Hope stressed that he believes that the term “False Positive” is a misnomer, and we must be extremely diligent to turn these findings into “True Negatives”.
Dr. Hope next addressed the important topic of whether PSMA PET imaging has an impact on disease management. In a study of 45 patients with high-risk disease at staging by Wu et al., 26 (53%) had changes in radiation therapy planning (12 received additional boost to the nodes, six had radiation therapy to bone metastases, and eight had nodal treatment outside the classic radiation template).4 A retrospective review by Boreta et al. of 125 patients with biochemical recurrence following radical prostatectomy and PSA <2.0 ng/ml who underwent 68Ga-PSMA PET imaging demonstrated that 66 (53%) of such patients had PSMA-avid disease. With standard intensity modulate radiation therapy templates, 30% of such men would have active nodal disease missed by such templates.5 Dr. Hope went on to present the data from Fendler et al. published in The Journal of Nuclear Medicine in 2019 that demonstrated that among 635 men with biochemical recurrence, management changes occurred in 68% of patients, with such changes considered major in 46%. The subgroup of such patients most affected by such major changes was the 0.5-2.0 ng/ml cohort. Patients with negative PET findings were subsequently managed with surveillance, those with pelvic nodal disease received radiation, and those with metastatic disease were given systemic hormonal therapy.6
The EMPIRE-1 trial, a single-center, open-label, phase 2/3 trial of 165 patients with biochemical recurrence following prostatectomy and negative conventional imaging were randomly assigned in a 1:1 Ratio to radiotherapy directed by conventional imaging alone or conventional imaging plus 18F-fluciclovine PET/CT. Patients in the interventional group (received both imaging modalities) had significantly improved biochemical recurrence-free survival at four years (75.5% versus 51.2%, p<0.001).7 Dr. Hope went on to introduce a trial of 68Ga-PSMA-11 PET/CT for prostate cancer salvage radiotherapy planning whereby 193 patients are evenly randomized to either salvage radiation therapy only or 68Ga-PSMA-11 prior to salvage radiation planning with a five year follow up planned.
Dr. Hope concluded his talk with the following three take-home messages:
- PSMA PET is superior to existing radiotracers for the detection of metastatic prostate cancer
- The use of PSMA PET has a large impact on clinical care, in particular with radiation therapy planning
- 68Ga-PSMA-11 and 18F-DCFPyL are approved by the FDA and are becoming widely available.
Presented by: Thomas Hope, MD, Assistant Professor, Abdominal Imaging and Nuclear Medicine, University of California-San Francisco, San Francisco, CA
Written by: Rashid Sayyid, MD, MSc – Urology Chief Resident, Augusta University/Medical College of Georgia, @rksayyid on Twitter during the 2021 Society of Urologic Oncology (SUO) Winter Annual Meeting, Orlando, FL, Wed, Dec 1 – Fri, Dec 3, 2021.
References:
- Calais J, Ceci F, Eiber M, et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019 Sep;20(0):1286-1294.
- Pienta KJ, Gorin MA, Rowe SP, et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol. 2021 Jul;206(1):52-61.
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021 Jul;27(13):3674-3682.
- Wu SY, Boreta L, Shinohara K, et al. Impact of Staging 68 Ga-PSMA-11 PET Scans on Radiation Treatment Plansin Patients With Prostate Cancer. Urology. 2019 Mar;125:154-162.
- Boreta L, Gadzinski AJ, Wu SY, et al. Location of Recurrence by Gallium-68 PSMA-11 PET Scan in Prostate Cancer Patients Eligible for Salvage Radiotherapy. Urology. 2019 Jul;165-171.
- Fendler WP, Ferdinandus J, Czernin J, et al. Impact of 68Ga-PSMA-11 PET on the Management of Recurrent Prostate Cancer in a Prospective Single-Arm Clinical Trial. J Nucl Med. 2020 Dec;61(12):1793-1799.
- Jani AB, Schreibmann E, Goyal S, et al. 18 F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): a single centre, open-label, phase 2/3 randomised controlled trial. Lancet. 2021 May;397(10288):1895-1904.


