(UroToday.com) The Society of Urologic Oncology (SUO) 2021 annual winter meeting included a prostate cancer session and a presentation by Dr. Andrew Armstrong discussing a post-hoc analysis of the ARCHES trial assessing the extended efficacy of enzalutamide plus ADT on oligometastatic hormone-sensitive prostate cancer. In ARCHES, enzalutamide + ADT reduced the risk of radiographic progression and improved secondary clinical outcomes in patients with metastatic hormone-sensitive prostate cancer (mHSPC) over placebo + ADT.1 A previous post hoc analysis demonstrated the efficacy of enzalutamide + ADT on bone-only oligometastatic disease but excluded soft tissue disease to control for the influence of variable metastatic spread. However, soft tissue metastases are often present in oligometastatic HSPC patients with or without bone metastases. Therefore, it is important to assess their influence on the disease and clinical outcomes. This extended post hoc analysis evaluated the efficacy of enzalutamide + ADT in patients with oligometastatic HSPC compared to polymetastatic HSPC in the ARCHES overall intent-to-treat (ITT) population, which included those with bone, soft tissue, or both types of metastases.
Patients with mHSPC (n=1150) were randomized 1:1 to enzalutamide (160 mg/day) + ADT or placebo + ADT, stratified by disease volume and prior docetaxel chemotherapy. Eligible patients in this post hoc analysis were those with bone (n=512), soft tissue (n=96), or both types of metastases (n=458), categorized as oligometastatic (1 – ≤5 metastases) or as polymetastatic (≥6 metastases) based on central review at screening. Efficacy outcomes were compared for patients on enzalutamide + ADT versus placebo + ADT within the defined groups and against polymetastatic disease. Additional analyses were conducted on patients without visceral disease to control for possible prognostic influence of visceral metastases (n=927). The primary endpoint was progression free survival (PFS), and secondary endpoints included OS, time to PSA progression, time to castration-resistance, time to first symptomatic skeletal event, time to initiation of new antineoplastic therapy, and PSA undetectable rate. All efficacy and safety data were reported with the data cut-off date of October 14, 2018 (median follow-up time, 14.4 months), except OS which was reported with a data cut-off date of May 28, 2021 (median follow-up time, 44.6 months).
Of the ARCHES ITT population with soft tissue, bone, or both types of metastases (n=1,066), the largest oligometastatic subgroup was patients with ≤5 metastases (enzalutamide + ADT, n=270; placebo + ADT, n=250). Baseline characteristics were generally comparable between treatment arms and across subgroups:

When compared to placebo + ADT, enzalutamide + ADT improved rPFS across all oligometastatic groups (HRs 0.26-0.46):

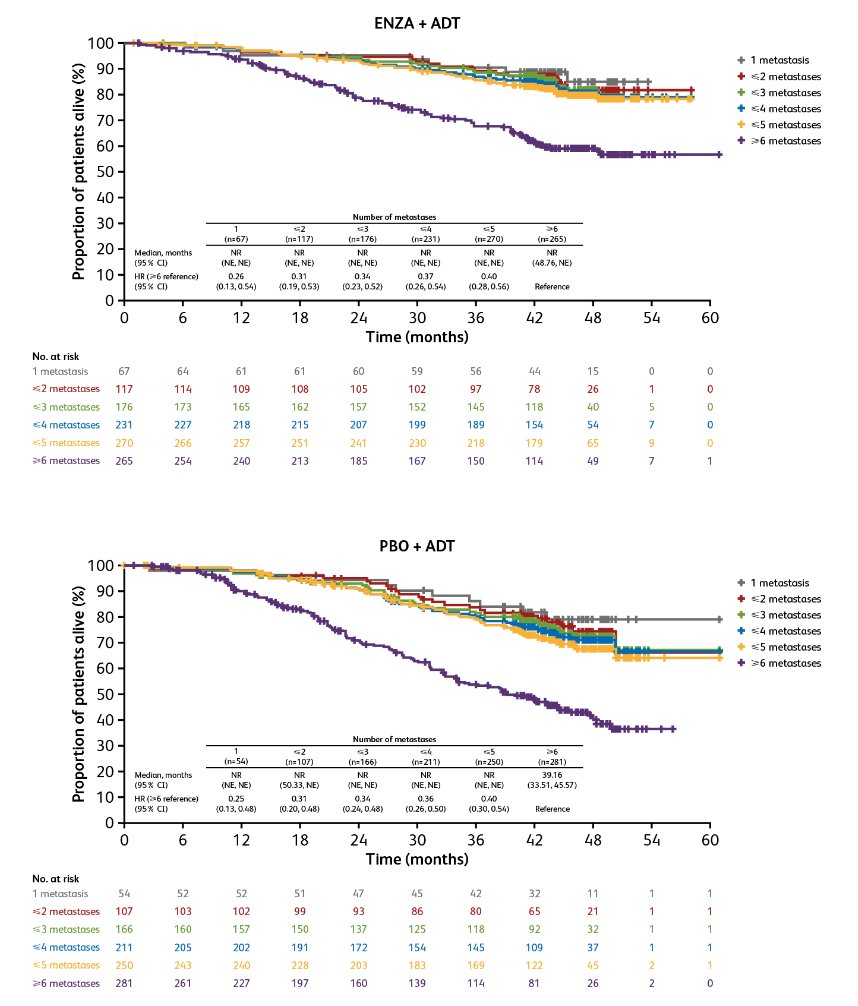
Similar findings favoring enzalutamide + ADT were found for improving OS (HRs 0.60-0.63):
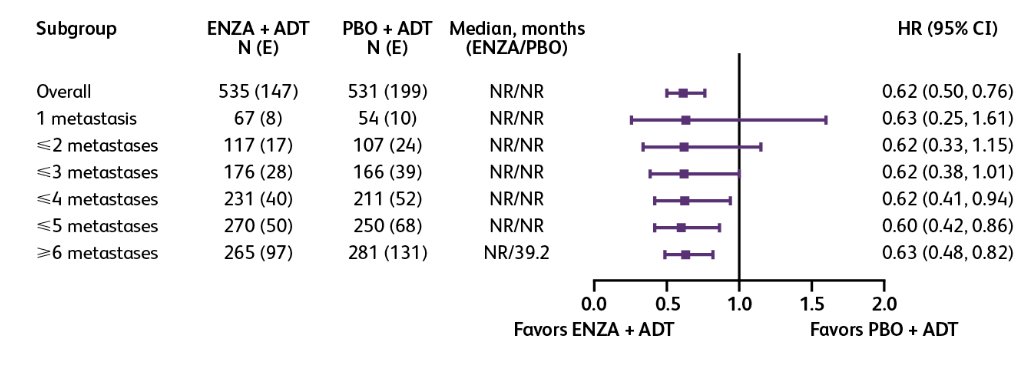
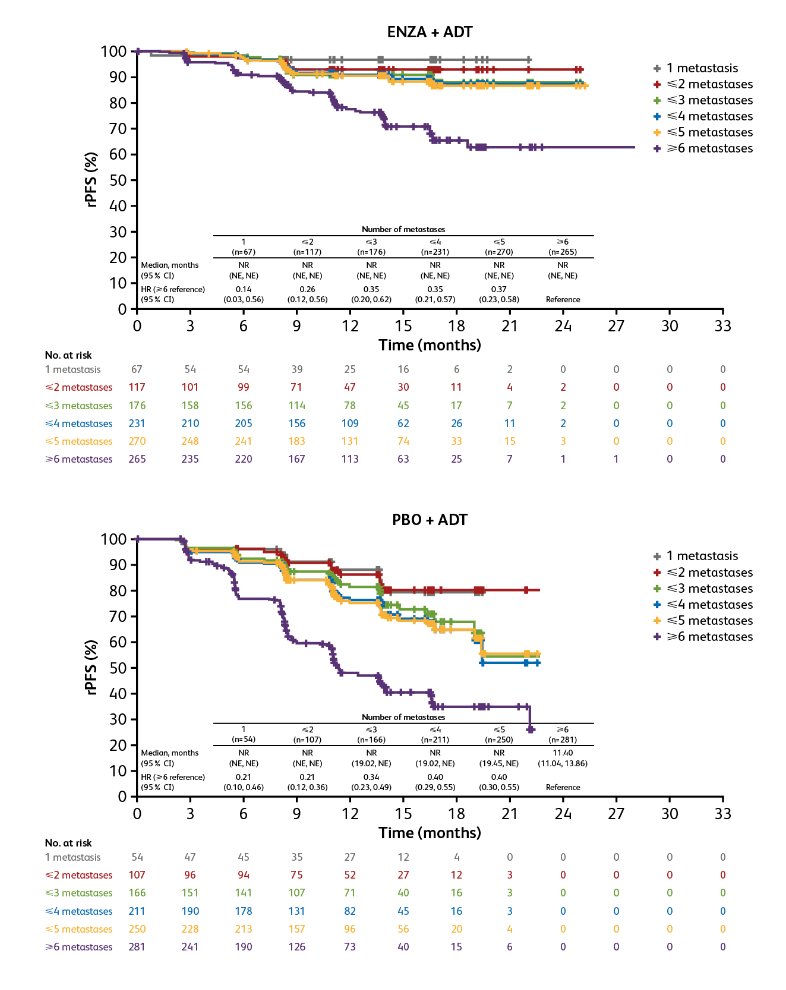
Patients with oligometastatic disease exhibited a better prognosis than those with polymetastatic disease in both the enzalutamide and placebo + ADT groups, regardless of the number of metastases used to define the group. HRs versus ≥6 metastases for rPFS ranged from 0.14-0.37 for enzalutamide + ADT and 0.21-0.40 for placebo + ADT:
Similarly, HRs versus ≥6 metastases for OS ranged from 0.26-0.40 for enzalutamide + ADT and 0.25-0.40 for placebo + ADT:
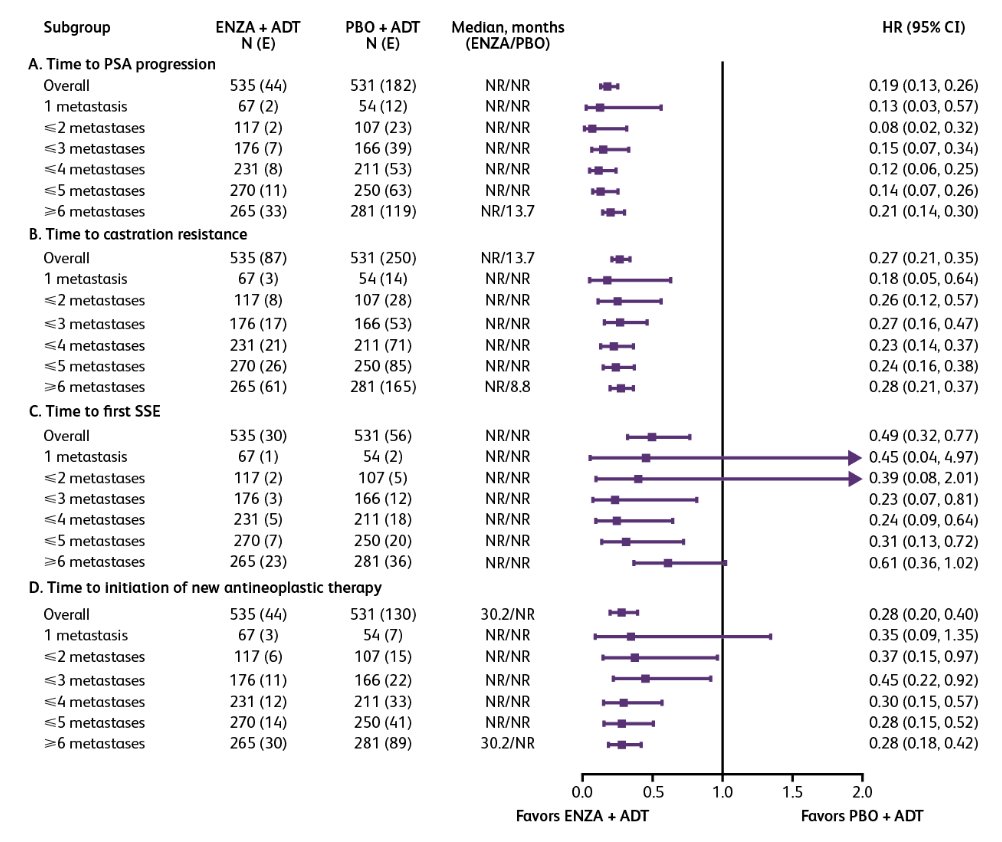
The treatment benefits of enzalutamide + ADT versus placebo + ADT in the oligometastatic and polymetastatic groups were also observed in additional key secondary endpoints:
Of the patients with detectable PSA at baseline, a greater proportion of those treated with enzalutamide + ADT achieved an undetectable PSA level (<0.2 ng/mL) than those treated with placebo + ADT in the oligometastatic and polymetastatic groups:

The safety profile of enzalutamide + ADT versus placebo + ADT was similar across subgroups and consistent with previous findings. In addition, the efficacy and safety profile observed in the ITT population oligometastatic and polymetastatic subgroups was maintained in patients without visceral metastases.
Dr. Armstrong concluded his presentation of extended follow-up of ARCHES with the following take-home messages:
- This post hoc analysis demonstrates that enzalutamide + ADT provided clinical benefit to patients with oligometastatic and polymetastatic HSPC, with bone, soft tissue, or both types of metastases
- Overall, these results validate and support previous findings observed in the bone-only oligometastatic ARCHES population and highlight the utility of enzalutamide irrespective of metastatic burden or type of oligometastatic disease in the ARCHES study
Presented by: Andrew J. Armstrong, MD, MSc, Duke Cancer Institute Center for Prostate & Urologic Cancers, Division of Medical Oncology and Urology, Durham, NC
Co-Authors: Jeffrey Holzbeierlein, Taro Iguchi, Arun A. Azad, Arnauld Villers, Boris Alekseev, Daniel P. Petrylak, Russell Z. Szmulewitz, Antonio Alcaraz, Neal D. Shore, Francisco Gomez-Veiga, Brad Rosbrook, Fabian Zohren, Gabriel P. Haas, Georgia Gourgioti, Nader N. El-Chaar, Arnulf Stenzl
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 Society of Urologic Oncology (SUO) Winter Annual Meeting, Orlando, FL, Wed, Dec 1 – Fri, Dec 3, 2021.
References:


