(UroToday.com) The Society of Urologic Oncology (SUO) annual winter meeting included the 2021 Richard D. Williams, MD Prostate Cancer Research Excellence Award Lecture with this year’s recipient being Dr. Gerald Andriole. As part of his receipt of this prestigious award, Dr. Andriole provided a discussion of considerations to improve screening in prostate cancer. Dr. Andriole started by highlighting that in the 2018 AUA prostate cancer early detection guidelines, recommendations for screening include (i) shared-decision making for men age 55-69 years of age, (ii) an interval of two years or more to reduce the harms of screening, and (iii) not recommending routine PSA screening for men more than 70 years of age, or for any man with a less than 10-15 year life expectancy. In the 2.2021 NCCN prostate cancer early detection guidelines, it states that clinicians should start the discussion of risks and benefits of prostate cancer early detection by obtaining a baseline PSA and strongly considering a baseline digital rectal examination.
Early work from Lilja et al.1 assessed the ability of a single PSA measured at age 44-50 to predict subsequent prostate cancer diagnosis in an unscreened population. This study included blood being collected from 21,277 men in a single Swedish city during 1974-1986 at ages 33-50. Through 2006, prostate cancer was diagnosed in 1,408 participants and PSA in archived plasma was measured for 1,312 of these cases (93%) and for 3,728 controls. In this population, ~75% of men had a PSA <1 at age 40-45. At a median follow-up of 23 years, baseline PSA was strongly associated with subsequent prostate cancer (AUC 0.72; 95% CI, 0.70-0.74; for advanced cancer, AUC 0.75; 95% CI, 0.72-0.78). Furthermore, 81% of advanced cases (95% CI, 77%-86%) were found in men with PSA above the median (0.63 ng/mL at ages 44-50).
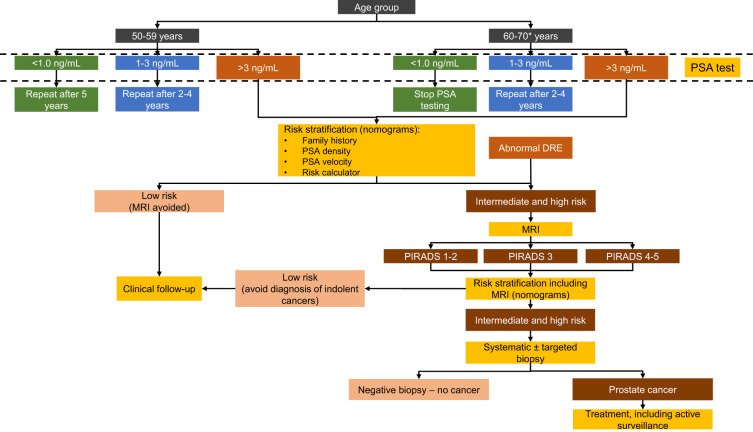
The EAU came out with similar recommendations this year for PSA as a part of risk-adapted early detection of prostate cancer,2 as highlighted in the following figure:
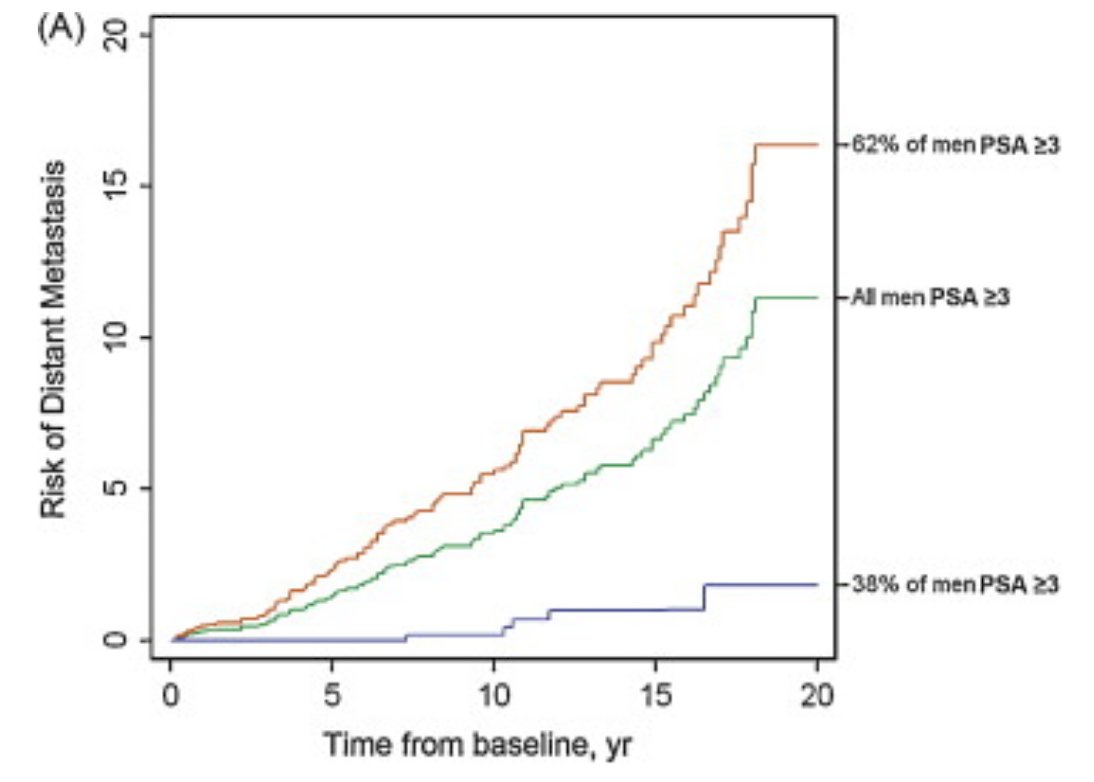
To attempt to increase the specificity of screening for lethal prostate cancer, Stattin et al.3 conducted a case-control study nested within a population-based cohort, measuring PSA and three additional kallikreins. Most metastatic cases occurred in men with PSA in the top quartile at age 50 years (69%) or 60 years (74%), whereas 20-yr risk of metastasis for men with PSA below median was low (≤0.6%). Among men with PSA >2 ng/ml, a prespecified model based on four kallikrein markers significantly enhanced the prediction of metastasis compared with PSA alone. As follows is the risk of distant metastasis among a 60-year old man with a PSA >= 3 ng/mL (green: overall risk of distant metastasis; orange: 4Kscore >=7.5%; blue 4Kscore <7.5%):
The PROBASE study (Risk-adapted prostate cancer early detection study based on a “baseline” PSA value in young men) is a German prospective multicenter, randomized trial. In this trial, men were randomized to receive a PSA at age 45 versus starting screening at 50 years of age. There are 23,301 men in the early arm, with 89% having a low PSA (<1.5 ng/mL) who will get a PSA every 5-years, 9% with moderate PSA (1.5-2.99 ng/mL) who will get a PSA every 2 years, and 1% with high PSA (>= 3 ng/mL) who will get an immediate MRI and prostate biopsy. So far, 0.19% of men have been found to have prostate cancer.
For the remainder of his talk, Dr. Andriole discussed 5 important points for improving screening for prostate cancer:
- Better identify which men are at above average risk
- Patients and primary care physicians need a simple message on PSA
- Identify patients with clinically significant prostate cancer earlier
- Reduce unnecessary initial and repeat prostate biopsies
- Enhance risk stratification: Better selection for surveillance versus interventional therapy
Better identify which men are at above average risk
Dr. Andriole notes that when talking to men about prostate cancer screening, it is important for each patient to understand their individual risk for prostate cancer, which is useful for informing them during shared-decision making. There are several important points to consider, including family history, African ancestry, baseline “early in life” PSA, reflex tests (blood: 4Kscore, PHI; urine: SELECT, PCA3, ExoDx), and combinations of the above. Other considerations include nomograms/risk calculators, hereditary risk (high penetrance genes, ie. BRCA; polygenomic risk score, ie PROMPT), and imaging (MRI, microultrasound, PET/MRI, or PET/CT).
Shi et al.4 previously published a study comparing the performance of polymorphism-based genetic risk score with two guideline-recommended inherited risk measures, family history and rare pathogenic mutations (BRCA2, HOXB13, and CHEK2), for predicting prostate cancer incidence and mortality. After a median follow-up of 9.67 years, 6,890 incident prostate cancer cases (419 died of prostate cancer) were identified. Each of the three measures was significantly associated with prostate cancer incidence in univariate analyses:
- Family history: RR 1.88 (95% CI 1.75-2.01)
- Rare pathogenic mutations: RR 2.89 (95% CI 1.89-4.25)
- Genetic risk score: RR 1.97(95% CI 1.87-2.07)
While family history and rare pathogenic mutations identified 11% of men at higher prostate cancer risk, the addition of genetic risk score identified an additional 22% of men at higher prostate cancer risk, and increases in C-statistic from 0.58 to 0.67 for differentiating incidence (p < 0.001) and from 0.65 to 0.71 for differentiating mortality (p = 0.002). As follows is the Venn diagram of high-risk men for prostate cancer identified with each of the three metrics:
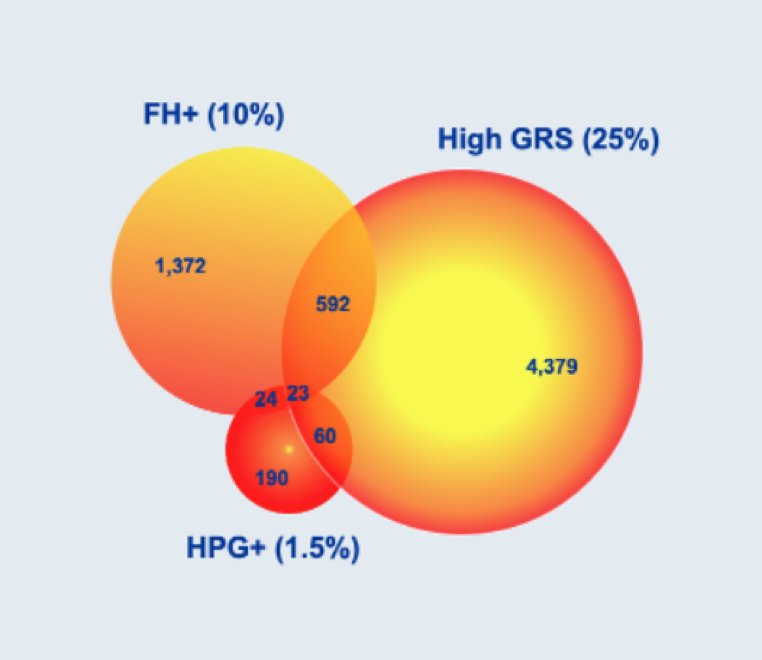
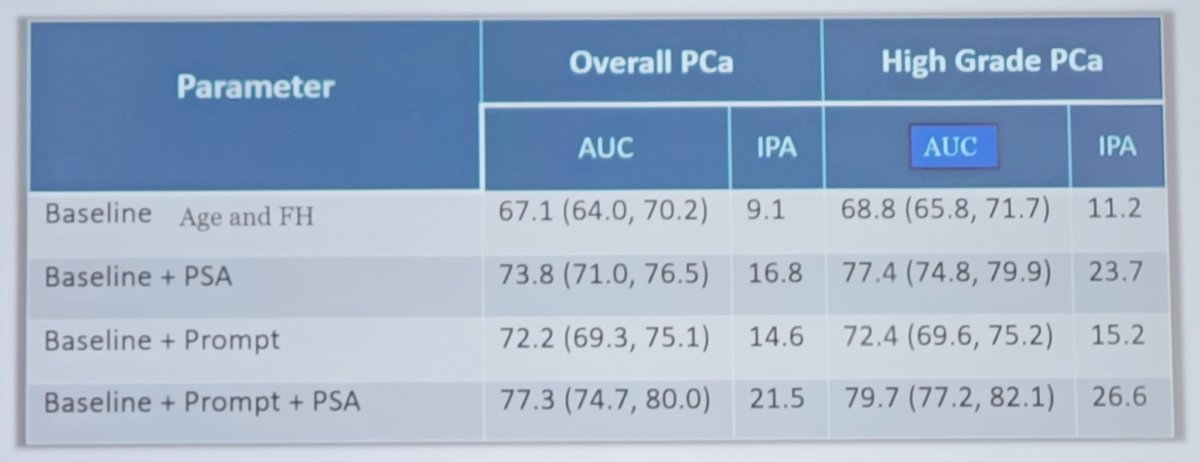
Furthermore, a baseline risk assessment including PROMPT-prostate genetic score improves the performance of PSA as highlighted in the following table:
Patients and primary care physicians need a simple message on PSA
Dr. Andriole notes that patients and primary care physicians are confused regarding the utility of PSA secondary to the USPSTF recommendations of Grade “D” for PSA screening in 2012, which has subsequently been increased to Grade “C”. Furthermore, there are multiple PSA cut-points that have been proposed and studied: (i) 2.5 ng/mL (Washington University for men <50 years of age), (ii) 3 ng/mL (European Screening Trial), (iii) 4 ng/mL (PLCO initial recommendations), (iv) “age-adjusted”, and (v) “race-adjusted”. According to Dr. Andriole, a possible solution is a dogma he has adopted from Dr. David Crawford in that PCPs should consider a PSA of 1-1.5 ng/mL as the threshold for referral to a urologist and then let him/her take it from there. Several studies have assessed the utility of a PSA of 1 ng/mL:
- PLCO: A PSA of <1 ng/mL is associated with a 0.5% risk of aggressive prostate cancer diagnosis in the next 10 years
- ERSPC: If a PSA is <1 ng/mL, the number needed to screen is 24,642 and the number needed to treat is 724 to prevent one death over 13 years
- Malmo: Stop screening if the PSA remains <1 ng/mL after age 60
In a 2020 publication from Kovac et al.5 looking at the association of a baseline PSA level with long-term diagnosis of clinically significant prostate cancer in men aged 55-60 in the PLCO trial, the 13-year Kaplan-Meier risk of prostate cancer stratified by PSA is as follows:
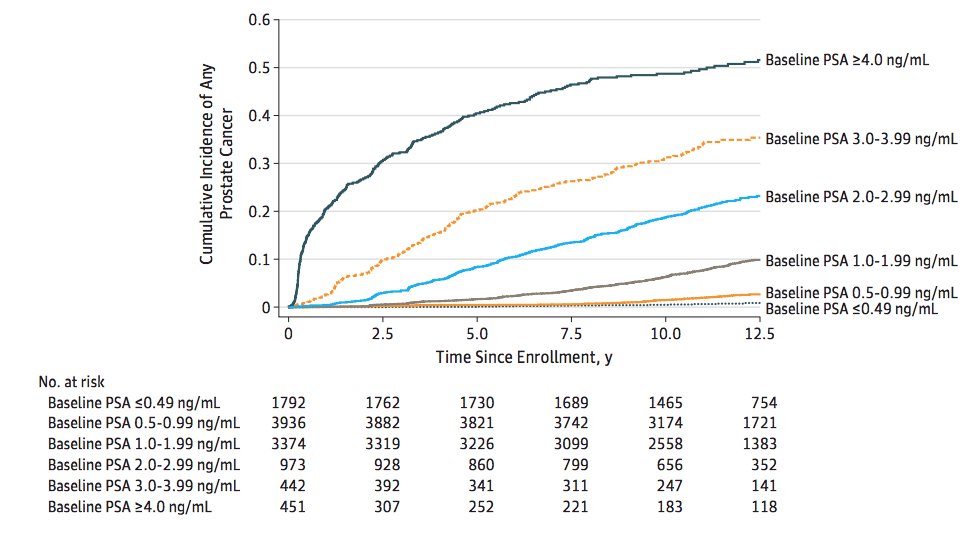
Identify patients with clinically significant prostate cancer earlier
Dr. Andriole highlighted that in the “best” screening trials, there is only a 20-30% relative risk reduction in prostate cancer mortality. Therefore, 70-80% of men destined to die of prostate cancer in the screened arm still died of prostate cancer despite being screened. We need to diagnose patients early with lower PSA cut-points and do better with prostate biopsies. As such, if a biopsy is warranted Dr. Andriole states that we need to do a “quality” biopsy. We should be avoiding conventional office-based, random, transfecal biopsies, given that it misses about half of cancers, and mischaracterizes many of the cancers it discovers. Image-guided (micro ultrasound or MRI) biopsies are preferred, and if not available template transperineal biopsies should be performed. Dr. Andriole notes that the negative predictive value for mpMRI across several series’ is not great, as highlighted as follows:
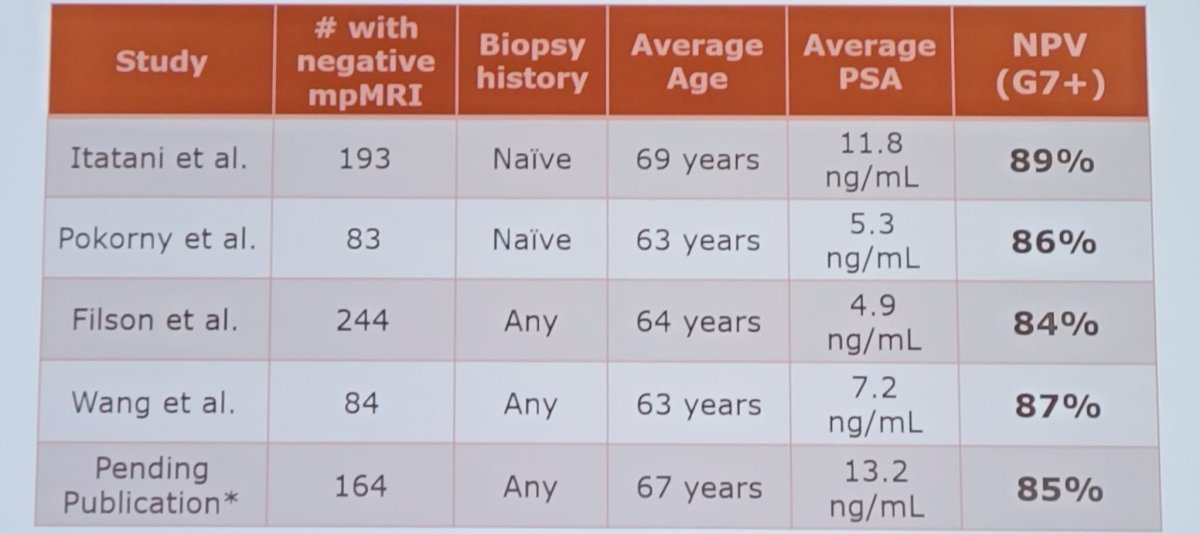
The novel micro-ultrasound system operates at 29 MHz, which is much higher than conventional 6-9 MHz systems. This results in a 300% improvement in resolution down to 70 microns, leading to image quality and detail comparable to that of an mpMRI and ability to see suspicious lesions. Thus, this enables real-time targeting of biopsies, which is completely controlled by the urologist. Several representative images are as follows:
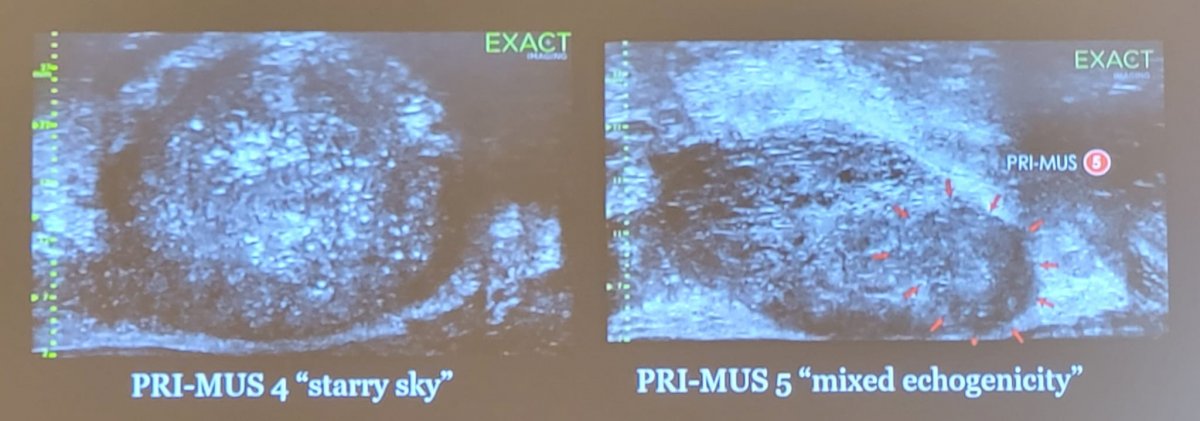
PRI-MUS is the classification used for micro-ultrasound assessment of lesions suspicious for cancer. Recently, this has been validated with positivity rates for GG>1 ranging from 4.4% (PRI-MUS 1) to 71.4% (PRI-MUS 5). Additionally, micro-ultrasound finds more cancer than conventional TRUS systematic biopsy, with the GG>1 rate increased by 12% (44.8% to 56.7%). Wiemer et al.6 evaluated micro-ultrasound of the prostate with real-time targeting of suspicious regions in 159 patients suspected to have prostate cancer. Prostate cancer was found in 113/159 (71%) men, with 49% (78/159) having clinically significant cancer (ISUP ≥ 2). Micro-ultrasound-targeted biopsies resulted in a higher ISUP grade group than the non-targeted biopsies in 26% (42/159), compared with both non-targeted and MRI-targeted biopsies in 16% (26/159). In 17% (27/159) of patients, targeted mpMRI-guided biopsy was negative with cancer identified in the micro-ultrasound-guided biopsy, of whom 20 had clinically significant prostate cancer. As follows is the distribution of grade groups depending on different biopsy strategy:
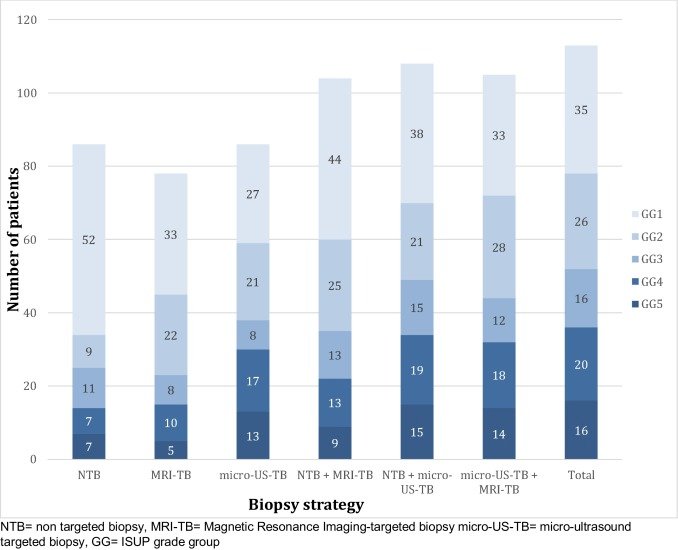
Dr. Andriole notes that he is part of the OPTIMUM (Optimization of prostate biopsy – Micro-ultrasound versus MRI) trial, which is set to open for enrollment in winter 2021. This is a 3-arm international RCT planned to provide level-1 evidence supporting the use of micro-ultrasound prostate biopsy. 1200 biopsy-naïve men will be randomized to micro-ultrasound only biopsy versus MRI/micro-ultrasound “FusionVu” biopsy versus MRI/US with conventional fusion system.
Reduce unnecessary initial and repeat prostate biopsies
According to the NCCN guidelines, patients should have an mpMRI if available prior to a prostate biopsy, and can consider biomarkers that improve the specificity of screening. Biomarkers to decide if a biopsy is necessary includes: free PSA (blood), ExoDx (urine), PHI (blood), OPKO 4K (blood), PCA3 (urine), and SELECT MDx (urine).
In a study presented at the 2019 AUA annual meeting, five-centers studied men who underwent a 4Kscore, mpMRI, and biopsy. Results from mpMRI were reported using PIRADS and categorized into low (PIRADS 1-2), intermediate (PIRADS 3) and high (PIRADS 4-5). The 4Kscore was reported as a continuous probability of high-grade GG2 or higher cancer, here categorized into low (<7%), intermediate (8%-32%), and high (>33%) risk. The primary endpoint was to evaluate potential Bx reduction, sensitivity, negative predictive value (NPV), and undetected high-grade cancers. The flow diagram for this study is as follows:
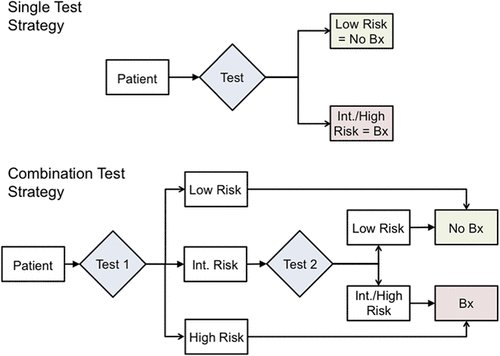
Of 1,120 men with a 4Kscore, 407 men with a 4Kscore, mpMRI and prostate biopsy were analyzed. Using a 4Kscore or MRI alone, resulted in a 25-27% biopsy reduction, with 8-12 men having an undetected high-grade cancer. Combination strategies yielded higher specificities, leading to larger biopsy reductions in the range of 38-41%, while 11-15 men had an undetected high-grade lesion. As such, combining 4Kscore and mpMRI to make prostate biospy decisions could further reduce biopsy rates, while improving specificity for high-grade cancer, with minimal changes to NPV and sensitivity.
Dr. Andriole also notes that it is time for “TRexit”: utilization of a transperineal grid or free hand transperineal biopsies. Previous studies have suggested feasibility of in-office ultrasound-guided transperineal prostate biopsy under local anesthesia using the PrecisionPoint transperineal access system. Furthermore, transperineal prostate biopsy improves the detection of clinically significant prostate cancer among men on active surveillance.
Enhance risk stratification: Better selection for surveillance versus interventional therapy
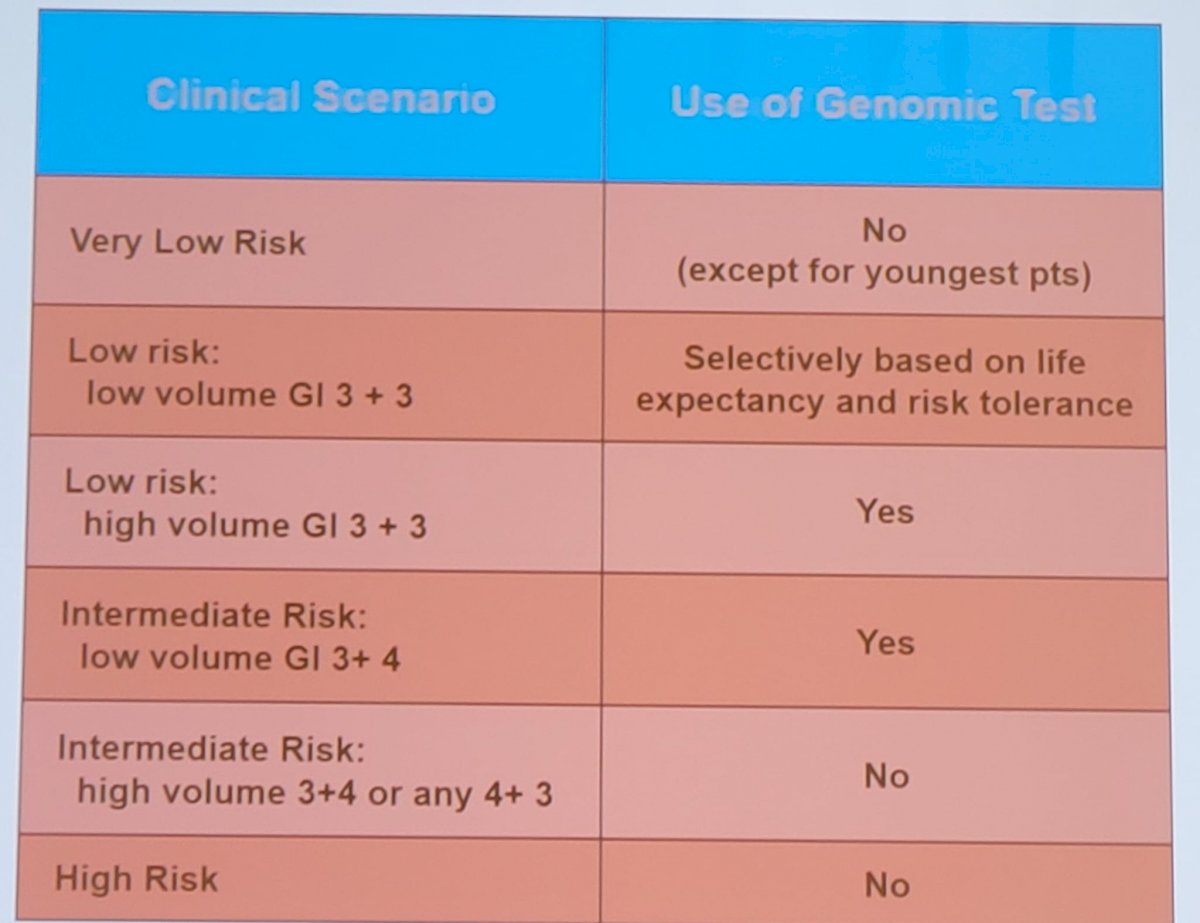
Dr. Andriole notes that genomic classifiers modestly lessen “over-treatment” of PSA-detected cancers. For favorable-risk patients, one additional patient undergoes active surveillance for every 9 men evaluated. For unfavorable risk patients, one patient out of 25 undergoes active surveillance instead of therapy. According to Dr. Andriole’s personal experience, use of genomic tests should be based on clinical scenarios as highlighted in the following table:
Dr. Andriole concluded his presentation of considerations to improve screening in prostate cancer with the following take-home messages:
- Aggressively screen those men who need it, based on family history, race, a baseline PSA in their 40’s, and genomic risk score
- Recommend early PSA cut-point for referral to Urology (~1.5 ng/mL)
- Abnormal PSA should not result in automatic biopsy get an MRI/micro-ultrasound and/or biomarker
- Do a quality biopsy, if needed, avoiding office-based, random, transfecal biopsies
- If cancer is detected, consider patient and tumor factors before recommending treatment
Presented by: Gerald L. Andriole, Jr, MD, Royce Distinguished Professor, Chief of Urologic Surgery, Washington University, St. Louis, MO
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 Society of Urologic Oncology (SUO) Winter Annual Meeting, Orlando, FL, Wed, Dec 1 – Fri, Dec 3, 2021.
References:
- Lilja H, Cronin AM, Dahlin A, et al. Prediction of significant prostate cancer diagnosed 20 to 30 years later with a single measure of prostate-specific antigen at or before age 50. Cancer. 2011 Mar 15;117(6):1210-1219.
- Van Poppel H, Roobol MJ, Chapple CR, et al. Prostate-specific Antigen Testing as Part of a Risk-Adapted Early Detection Strategy for Prostate Cancer: European Association of Urology Position and Recommendations for 2021. Eur Urol. 2021 Dec 1 [Epub ahead of print].
- Stattin P, Vickers AJ, Sjoberg DD, et al. Improving the specificity of screening for lethal prostate cancer using prostate-specific antigen and a panel of Kallikrein Markers: A Nested Case-Control Study. Eur Urol. 2021 Aug;68(2):207-213.
- Shi Z, Platz EA, Wei J, et al. Performance of Three Inherited Risk Measures for Predicting Prostate Cancer Incidence and Mortality: A Population-based Prospective Analysis. Eur Urol. 2021 Mar;79(3):419-426.
- Kovac E, Carlsson SV, Lilja H, et al. Association of Baseline Prostate Specific Antigen level with long-term diagnosis of clinically significant prostate cancer among patients aged 55 to 60 years: A secondary analysis of a cohort in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. JAMA Net Open. 2020;3(1):e1919284.
- Wiemer L, Hollenbach M, Heckman R, et al. Evolution of targeted prostate biopsy by adding micro-ultrasound to the magnetic resonance imaging pathway. Eur Urol Focus. 2020 Jul 9;S2405-4569(20)30188-7.


