(UroToday.com) In a plenary session of the Society of Urologic Oncology Annual Meeting focused on transperineal prostate biopsy, Dr. Badar Munir Mian discussed current trials assessing the role of transperineal biopsy in prostate cancer.
He began by emphasizing that, currently, there is a paucity of data comparing transperineal and transrectal biopsy and thus, the evidentiary base remains uncertain. Available data are typically case series and case reports, supplemented by some expert opinion but typically relying on a single-arm design without controls and with highly variable regimes including differing antibiotic prescribing approaches and biopsy templates. As a result, data are inconsistent and often contradictory. Further, differences between transperineal and transrectal biopsy approaches are, where present, often weak and of minor magnitude.
Despite the limitations of the available data, Dr. Mian emphasized that the EAU has published a position paper highlighting that transperineal biopsy should be the “1st choice” where it is feasible.
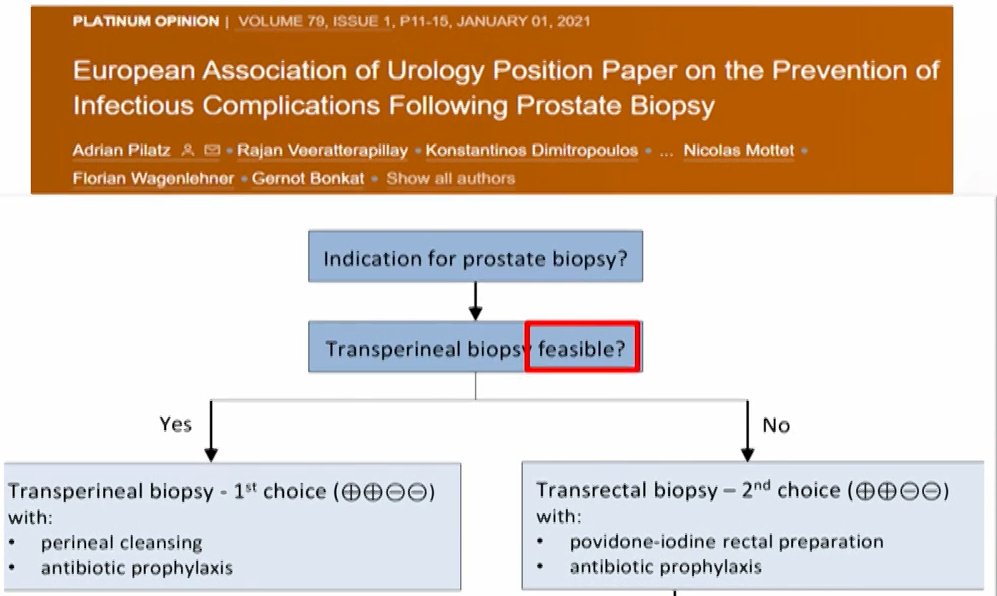
However, he highlighted that this position paper did not recommend AGAINST transrectal biopsy. In supporting this recommendation, he highlighted data from a recent systematic review and meta-analysis of 7 RCTs assessing non-antibiotic strategies to reduce infectious complications. This study showed a lower rate of infection following transperineal biopsy (3.3%) compared to transrectal biopsy (5.6%; difference = 2.3%, p=0.02). However rates of hospitalization were similar and the authors assessed that there was a high risk of bias, thus leading to a low-GRADE recommendation. Additionally, the National Prostate Cancer Audit of England provides informative data. Among more than 70,000 patients undergoing biopsy, there was a substantially higher rate of sepsis admissions for patients undergoing transrectal biopsy (1.4%) than transperineal biopsy (1%) though this was weighted against higher admissions due to urinary retention in the transperineal group (1.9% vs 1%). Thus, while the number needed to treat with transperineal biopsy to avoid 1 sepsis admission was 278, this was associated with 3 additional hospitalizations for urinary complications. Additionally, patients undergoing transperineal biopsy were more likely to require an overnight stay (12.3% vs 2.4%). Finally, Dr. Mian cited data from a recent review of 165 studies encompassing 162,577 patients which found that there were no statistically significant differences between the approaches with respect to hospitalizations, sepsis, or urinary retention, though absolute rates differed.

Given all these data, the EAU Position Paper acknowledges that “data is of low certainty… our confidence in the effect estimate is limited”. Thus, he described current enthusiasm towards transperineal biopsy as “irrational exuberance”.
Dr. Mian emphasized that we need adequately powered and well-designed randomized controlled trials which take into account contemporary concerns including the use of MRI-fusion with both biopsy approaches, local anesthesia, and the avoidance of antibiotics. To date, there are no such published studies though three are ongoing.
He first highlighted that TRANSLATE trial which is being performed in the UK and seeks to enroll 1100 patients who are biopsy naïve with MRI based suspicion of prostate cancer. The study is powered to primarily assess prostate cancer detection rates with infectious complications as a secondary outcome. This trial is starting imminently.
In the US, NCT04815876, led by Dr. Jim Hu, initially planned to enroll 400 biopsy naïve patients with an expected completion date of June 2025. Subsequently, with funding from PCORI, a related trial was expanded to 1302 patients at 12 sites and included those on active surveillance and undergoing repeat biopsies. The outcomes of interest include infectious complications as well as cancer detection. The trial is now ongoing and expected to be complete in April 2025.
Finally, he highlighted the ongoing PROBE-PC trial from Albany Medical College which, starting in July 2019, is enrolling 600 patients with the primary outcome of infectious complications. This trial includes patients undergoing prostate biopsy whether biopsy naïve, for repeat biopsy, or on active surveillance. The primary outcome of infectious complications within 30 days of biopsy includes sepsis, urinary tract infection, fever, perineal cellulitis or infection, antibiotic prescriptions, and asymptomatic bacteriuria, regardless of culture results. There are additional secondary outcomes including pain, cancer detection, healthcare utilization, bleeding complications, urinary and sexual function, and cost.
To date, this trial has randomized 547 of whom 510 have completed and the trial is expected to close in 3-4 months.Presented by: Badar Munir Mian, MD, FACS, Albany Medical Center


