(UroToday.com) The second Prostate Cancer Session of the Annual Meeting of the Society of Urologic Oncology was held on Friday, December 2nd, 2022. In this session, Dr. Ana Aparicio presented on the role of biomarkers and somatic testing to guide treatment choice in advanced prostate cancer.
Dr. Aparicio noted that, while sharing many similarities, there are important differences between predictors of tumor response to specific therapies and classifiers of biologic phenotypes. She noted that specific features, including homologous recombination repair deficiency, microsatellite instability (MSI-high), and high prostate-specific membrane antigen (PSMA) expression are predictive of response to PARP inhibitors, pembrolizumab, and 177Lu-PSMA-617, respectively. However, Dr. Aparicio noted specific potential limitations with applying each of these to clinical practice.

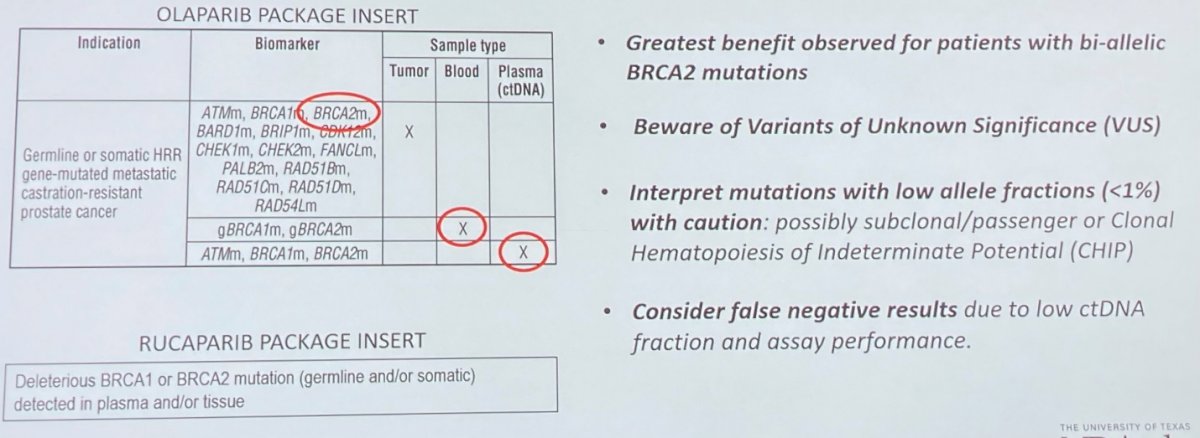
She first focused on the role of homologous recombination repair (HRR) gene mutations and PARP inhibition in patients with metastatic castration-resistant prostate cancer (mCRPC). As identified in the product monographs, the identification of these variants is critical for eligibility for these therapies. However, stepping through to clinical care, there are potential problems. She noted that while patients with mutations in one of 15 HRR genes are potentially eligible for treatment with olaparib under the correct clinical setting, the greatest benefit of therapy is realized in patients with bi-allelic loss of BRCA2 function. In many cases, she noted that testing results may not provide this critical information to distinguish mono-allelic from bi-allelic loss.
She further highlighted the importance of considering variants of unknown significance. These may represent up to half of all identified mutations. Where plasma (ctDNA) based testing is performed, she noted that we must interpret mutations with a low allelic frequency (<1%) with caution. However, many testing reports do not provide this information. This is important as identified variants may actually represent subclonal or variant mutations with minimal clinical relevance, or clonal hematopoiesis of indeterminate potential (CHIP). Further, in this ctDNA setting, she noted that we should consider the potential for false negative results as a result of either low ctDNA fraction or assay performance.
She then moved to discuss the importance of microsatellite instability in the context of PD-1 inhibition. In keeping with the FDA approval and resulting product monograph, patients with microsatellite instability-high (MSI-high) or mismatch repair deficient tumors or high tumor mutational burden may be eligible for treatment with pembrolizumab, regardless of primary tumor site. In the context of mismatch repair deficiency, she noted that immunohistochemistry (IHC) or next generation sequencing (NGS) may be undertaken to identify aberrations in MLH1, MSH2, MSH6, and PMS2. However, she noted that normal staining may occur despite deleterious mutations as a result of the presence of non-functional mutations. Further, as alluded to above in the context of HRR deficiency, variants of unknown significance and CHIP may affect interpretation. Microsatellite instability may be detected by either PCR or NGS. However, she noted that there may be substantial variation in instability of microsatellite loci between cancer types in terms of both size and number. In the context of tumor mutational burden, NGS is typically employed through targeted panels may overestimate TMB due to enrichment for cancer genes in these panels.

Dr. Aparicio then noted that there has been some recent progress in terms of the development of classifiers. Certainly, in terms of the “eye-ball test”, many clinicians can identify patients for whom the disease process is more or less driven by the androgen axis on the basis of clinical presentation. Among those who are least androgen driven, she noted that patients with small cell or neuroendocrine prostate cancer may be diagnosed histologically, though should not depend on IHC of “neuroendocrine” markers. While this entity is relative uncommon at initial diagnosis (<1%), under the selective pressures of androgen deprivation, this may emerge in up to 10% of patients. These tumors are molecularly distinct from other subgroups of prostate cancer, with notable low AR activity and high neuroendocrine marker expression. While resistant to androgen signalling inhibition, these tumors are relatively sensitive to platinum-based chemotherapies.
Moving somewhat more broadly, she noted aggressive variant prostate cancer (AVPCs), a subset of heterogenous morphologies that share the virulent, atypical clinical features of neuroendocrine prostate cancer and are characterized by combined tumor suppressor defects. In terms of molecular profile, most of these tumors have mutations in at least two of p53, RB1, and PTEN.
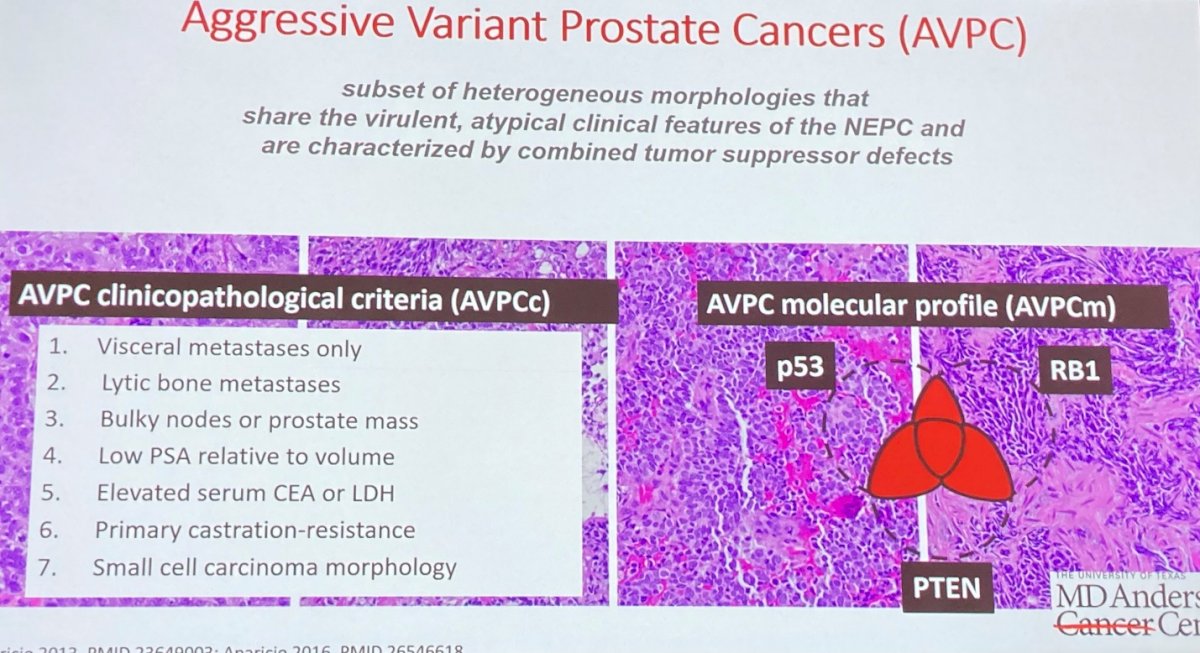
These patients may benefit from the addition of carboplatin to cabazitaxel, based on randomized phase II data. While there are shared characteristics within AVPCs, Dr. Aparicio noted that transcriptional profiling demonstrates substantial heterogeneity including at least 3 different molecular phenotypes.
In summary, Dr. Aparicio noted that HRR, MSI, and PSMA uptake are predictive of benefit from specific treatment approaches. Ongoing improvements in assays for detection of these markers is anticipated. However, development of classifiers to guide therapeutic strategies (including combination approaches and sequencing) remain a critically needed area of research.
Presented by: Ana Aparicio, MD, Professor, Department of Genitourinary Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston


