(UroToday.com) The 2023 SUO annual meeting included a session on the management of complete clinical response following neoadjuvant systemic therapy for muscle invasive bladder cancer, featuring a presentation by Dr. Anne Schuckman discussing that radical cystectomy should be performed after achieving cT0 after neoadjuvant chemotherapy.
Dr. Schuckman notes that the current standard of care for muscle invasive bladder cancer is neoadjuvant chemotherapy with a cisplatin containing regimen followed by consolidative radical cystectomy + lymph node dissection. This standard of care was based on the SWOG 8710,1 which showed an improvement in overall survival with the addition of chemotherapy, driving the push for a new standard of care, despite likely overtreatment in many patients. Benefit for these patients is derived from treatment of micro-metastatic disease and increasing the rate of pT0. However, there are potential arguments for omitting radical cystectomy, and include:
- “Comparable outcomes” without radical cystectomy in cT0 patients
- Avoid the morbidity of surgery and urinary diversion
- No worse outcomes with delayed management
- Deintensification of treatment/quality of life
That withstanding, there are several important questions regarding the possibility of potentially omitting radical cystectomy:
- Does cT0 = pT0?
- Can we use biomarkers to predict response to chemotherapy?
- Is salvage/delay radical cystectomy equal to upfront cystectomy?
- Are we under-treating metastatic disease?
- What is the financial and emotional stress/toxicity of ongoing surveillance versus radical cystectomy?
pT0 has been used as a surrogate endpoint for the success of neoadjuvant chemotherapy, again taking data from the SWOG 8710 trial.1 Patients that achieved pT0N0 (pathologic complete response) had a 5 year overall survival rate of 88%, a 26% lower absolute risk of mortality, and a 51% absolute lower risk of recurrence compared with pathological residual disease. Furthermore, the overall survival for ypT>0 was 3.4 years versus 11 years for ypT0. At her institution (USC), Dr. Schuckman notes that the natural history of ypT0/pT0N0 (n = 234, 2000-2019) is a recurrence free survival rate of 85%/84% for ypT0 and 99%/95% for pT0.2
Dr. Schuckman posed the question: Since we have ample evidence that patients who are TRULY ypT0 do well, can we identify them prior to radical cystectomy? Unfortunately, the rate of accurate clinical staging is quite variable and poor based on traditional staging (CT or other cross sectional imaging, TURBT after chemotherapy, physical examination, and cytology):
- MD Anderson Cancer Center: 35% correct rate of ypT0, 25% >= T3 or N+ (12.7% N+)
- SWOG 0219: 40% correct rate of ypT0
- Multi-institutional cohort: 47% correct rate of pT0, 43% >= T2 and 12% N+
- Columbia University: 66% correct rate of pT0
The TUR accuracy after neoadjuvant chemotherapy was assessed in the Systematic Endoscopic Evaluation Trial at the Fox Chase Cancer Center, whereby a biopsy of visible tumor, the tumor bed, and random biopsies were performed prior to radical cystectomy.3 Among 61 patients enrolled in the trial, 38 (62.3%) received neoadjuvant chemotherapy. On Systematic Endoscopic Evaluation, 31 (50.8%) patients demonstrated no visual nor biopsy-based evidence of disease (seeT0), yet 16/31 (51.6%) harbored residual disease (>pT0), including 8 (8/31, 25.8%) with residual ≥pT2 disease upon radical cystectomy. The negative predictive value of Systematic Endoscopic Evaluation predicting a pT0 bladder was 48.4% (CI 30.2-66.9), below the prespecified hypothesis.
There is also an inaccuracy of radiological staging for these patients. In a study to assess MRI without versus with intravenous contrast (biparametric vs. multiparametric) for identifying residual disease on cystectomy, Woo and colleagues found that among 61 patients, biparametric MRI was more accurate than multiparametric MRI for detecting residual disease after NAC: AUC 0.75 vs. 0.58 (p = 0.043).4 The sensitivity was identical (65.1%) but specificity was higher in biparametric MRI compared with multiparametric MRI for determining residual disease: 77.8% vs. 38.9%, respectively. Concordant positive (MRI+/pathology+) patients showed worse disease free survival than concordant negative (MRI-/pathology-) patients (HR 8.75, 95% CI, 2.02-37.82) and compared to the discordant group (MRI+/pathology- or MRI-/pathology+) with HR 3.48 (95% CI, 1.39-8.71):
Data from the PURE-01 study,5 assessing 82 patients with 164 total multiparametric MRIs, showed that the agreement between the internal and external mpMRI assessments after therapy was acceptable (κ values ranging from 0.5 to 0.76). The AUC was 0.74 for residual disease, pT0 showed 62-73% accuracy, pT<=1 showed 95% accuracy, with 20% inter-observer disagreement: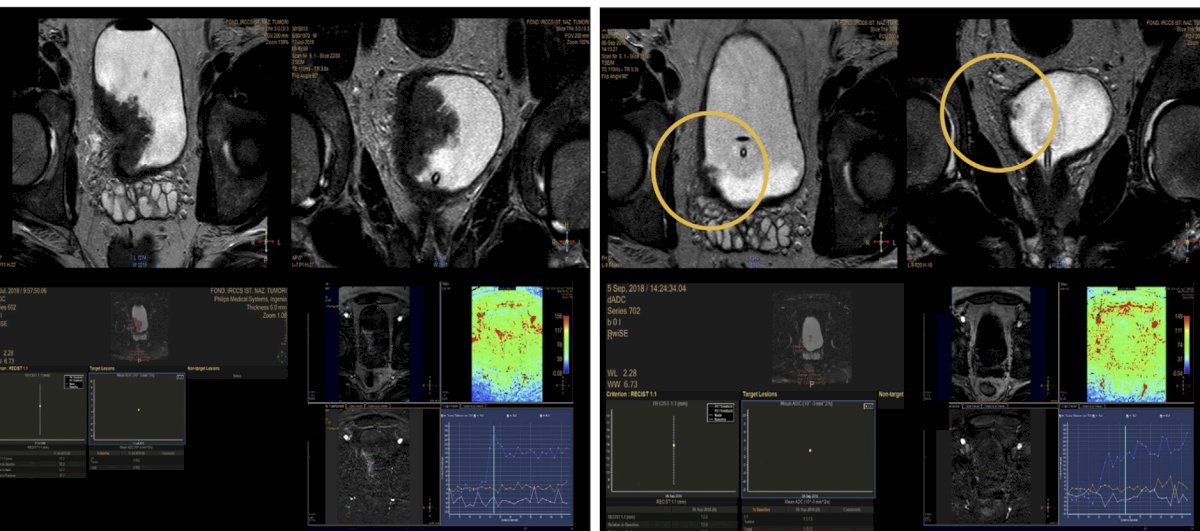
With regards to FDG PET staging, results have generally been disappointing with a 70% sensitivity and specificity for tumor pathologic complete response, and a 67% sensitivity and specificity for overall pathologic complete response. In a study assessing nacVI-RADS, among 10 patients, nac-VI-RADS categories were able to match all the final radical cystectomy pathology for complete responders, as well as match all partial responders with inter-class downstaging. To summarize, Dr. Schuckman notes that the overall accuracy of current clinical staging is 60-70% at best using current TUR and imaging modalities.
Can we use biomarkers to predict response to chemotherapy? Perhaps VI-RADS prediction will continue to evolve in our ability to predict response to neoadjuvant chemotherapy. Perhaps there will be emergence of a molecular signature or ctDNA for selection of bladder preservation? MRI radiomics suggests that qRECIST has an AUC of 0.91 and 75% accuracy in determining responders from non-responders, with an ideal situation being a prediction of response to chemotherapy after only 1-2 cycles of neoadjuvant chemotherapy: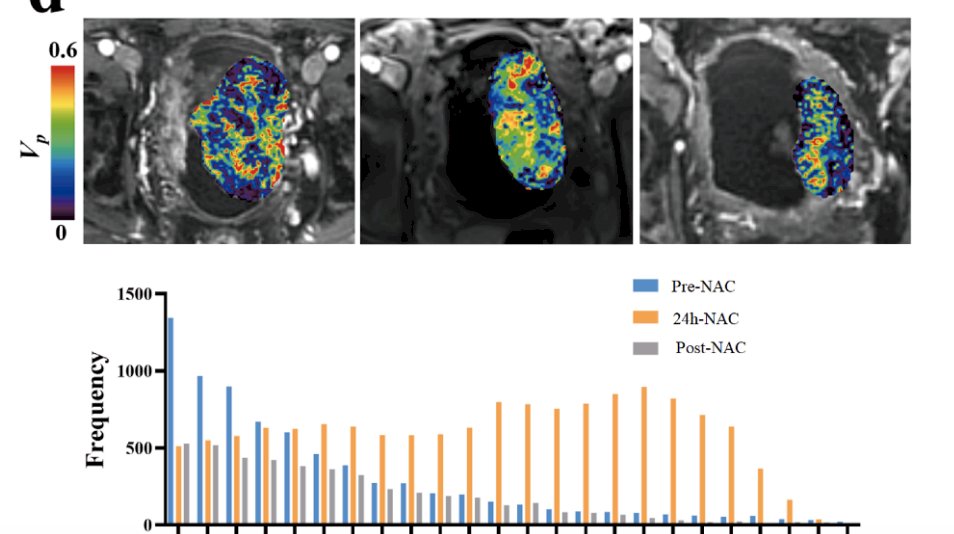
Dr. Schuckman then discussed RETAIN I, a phase II, multi-institutional, non-inferiority trial to evaluate a risk-adapted approach to the treatment of muscle invasive bladder cancer. Pre-chemotherapy, the primary tumor is sequence for ATM, ERCC2, FANCC, or RB1, then the patient is treated with 3 cycles of neoadjuvant aMVAC followed by a restaging TURBT and the following post-chemotherapy options:
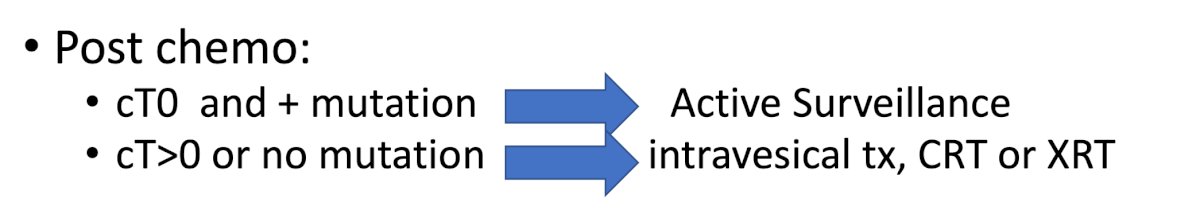
The primary endpoint of the trial is metastasis free survival at 2 years. An update on RETAIN was presented at GU ASCO 2023 by Dr. Daniel Geynisman. From 4 academic centers, 102 patients were enrolled over 33 months, with the ITT cohort ultimately being 71 patients. The median age was 70 years (IQR: 47-83), 74% were male, 92% Caucasian, 81% were ECOG PS 0, and 79% had cT2 disease. Overall, 90% completed 3 cycles of MVAC with 17% grade 3-4 TRAEs. Among the 37 patients who were mutation negative, most went on to cystectomy, though two did opt for active surveillance, six for chemoradiation, and three for BCG. In the ITT population, 33 (46%) had a relevant mutation and 26 (37%) began active surveillance. With a median follow-up of 41 months, 47 patients (66%) are metastasis-free (CI 54%-77%). The 2-year MFS for the ITT patients was 72% (lower bound exact 1-sided 95% CI = 62%). Unfortunately, this did not meet predefined cutoff for significance, thus the trial could not declare the risk-adapted approach non-inferior. On post hoc analysis, the 2-year MFS was 76.9% in the AS group and 70.5% in the remaining patients (no significant difference).
There are several trials that are planned or ongoing for bladder preservation in muscle invasive bladder cancer: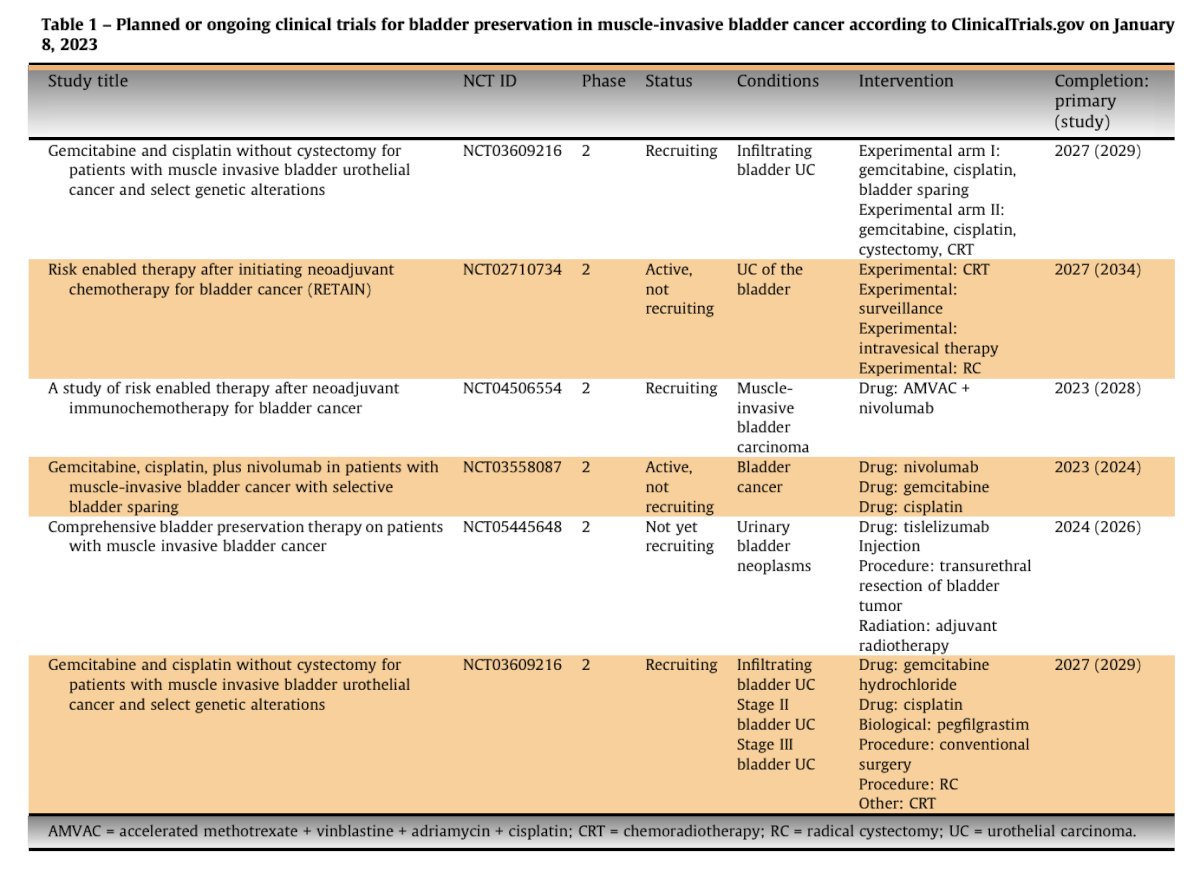
With regards to whether salvage/delayed radical cystectomy is equivalent to upfront radical cystectomy, the literature suggests that delaying cystectomy in these patients likely leads to worse outcomes, with one study suggesting a 30% excess disease mortality for delayed surgery: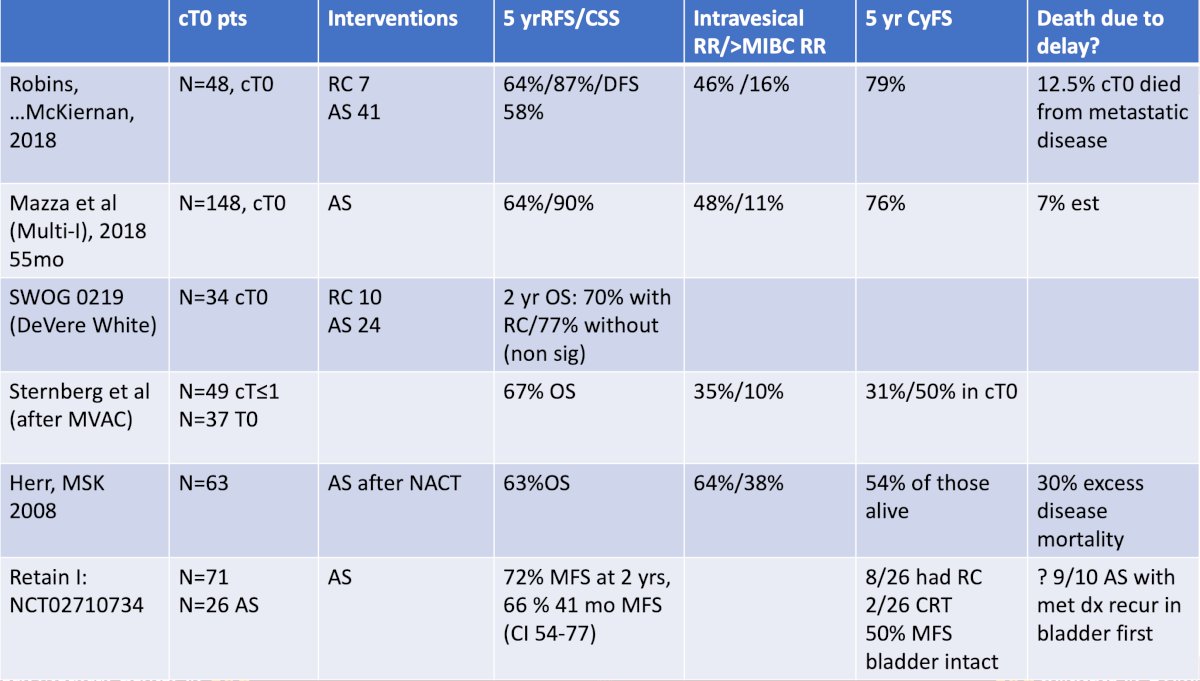
Generally, based on the traditionally available criteria, the patients who do well with bladder preservation are as follows:
- Initially solitary T2 tumors
- No CIS at any point in time
- No hydronephrosis
- No lymphovascular invasion
- Small tumors
Are we under-treating metastatic disease? Dr. Schuckman then discussed the role of the extent of lymphadenectomy and survival among patients with lymph node metastases undergoing radical cystectomy. Wright and colleagues noted that in patients undergoing lymph node dissection for node positivity, there was an increase in overall survival. Additionally, a lymph node yield of >10 correlated with a significant increase in overall survival.6
Finally, what is the financial and emotional stress/toxicity of ongoing surveillance versus radical cystectomy? Patients who retain their bladder require ongoing need for cystoscopic surveillance, as well as increased financial toxicity of surveillance and systemic maintenance therapy. Moreover, there is always the looming threat of salvage therapy and the bladder toxicity associated with multiple intravesical therapies. Dr. Schuckman notes that non-muscle invasive bladder cancer is more expensive than muscle invasive bladder cancer due to longer follow-up, and we are now potentially stacking the cost of muscle invasive bladder cancer and non muscle invasive recurrences: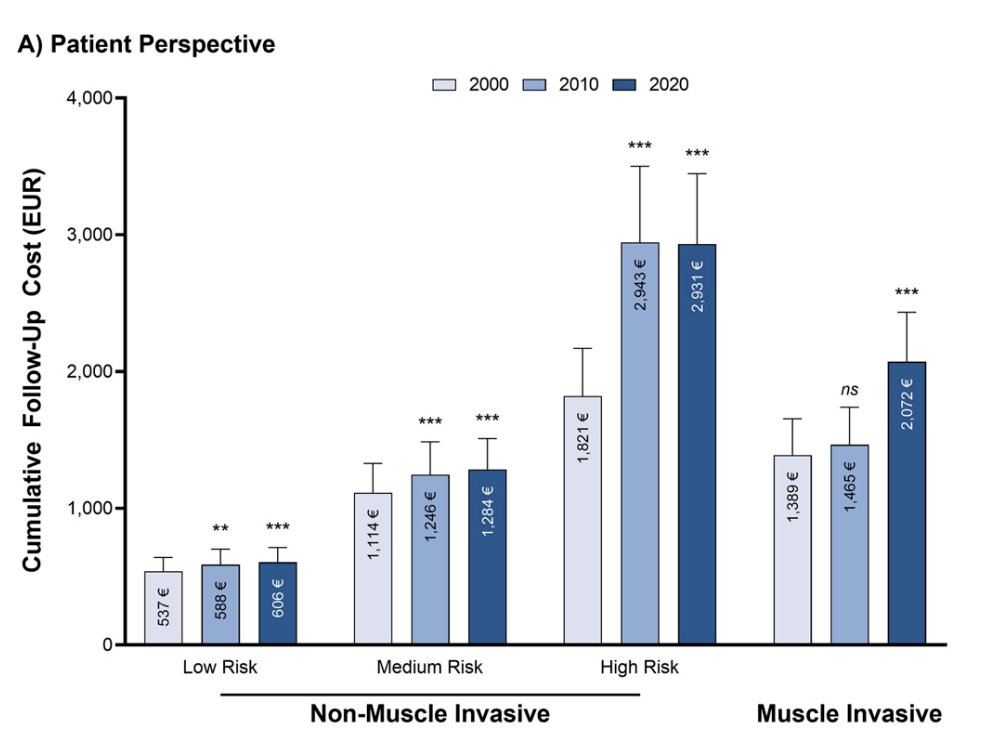
Dr. Schuckman concluded her presentation discussing neoadjuvant chemotherapy followed by radical cystectomy despite a complete clinical response in the setting of muscle invasive bladder cancer with the following take-home points:
- Radical cystectomy allows accurate staging and prognostication
- Radical cystectomy allows timely delivery of necessary adjuvant therapies
- There is a 2-4% 30-90 day mortality risk associated with radical cystectomy
- Clavien Grade >3 complications associated with radical cystectomy are 15%
- With appropriate selection, we may save up to 10% additional mortalities related to urothelial carcinoma in neoadjuvant chemotherapy responders
Presented by: Anne K. Schuckman, Keck School of Medicine, University of Southern California, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 Society of Urologic Oncology (SUO) Annual Meeting, Washington, D.C., Tues, Nov 28 – Fri, Dec 1, 2023.
References:
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349(9):859-866.
- Douglawi A, Ghoreifi A, Lee R, et al. Recurrence patterns in bladder cancer patients with no residual disease (pT0N0) at radical cystectomy: A 20-year experience. Urol Oncol. 2023 Feb;41(2):107.e9-107.e14.
- Zibelman M, Ashgar AM, Parker DC, et al. Cystoscopy and systematic bladder tissue sampling in predicting pT0 bladder cancer: A prospective trial. J Urol. 2021 Jun;205(6):1605-1611.
- Woo S, Becker AS, Das JP, et al. Evaluating residual tumor after neoadjuvant chemotherapy for muscle-invasive urothelial bladder cancer: Diagnostic performance and outcomes using biparametric vs. multiparametric MRI. Cancer Imaging. 2023 Nov 14;23(1):110.
- Necchi A, Bandini M, Calareso G, et al. Multiparametric Magnetic Resonance Imaging as a Noninvasive Assessment of Tumor Response to Neoadjuvant Pembrolizumab in Muscle-invasive Bladder Cancer: Preliminary findings from the PURE-01 Study. Eur Urol. 2020 May;77(5):636-643.
- Wright JL, Lin DW, Porter MP. The association between extent of lymphadenectomy and survival among patients with lymph node metastases undergoing radical cystectomy. Cancer. 2008 Jun;112(11):2401-2408.


