(UroToday.com) The 2023 SUO annual meeting included a session on the management of complete clinical response following neoadjuvant systemic therapy for muscle invasive bladder cancer, featuring a presentation by Dr. James McKiernan discussing neoadjuvant chemotherapy followed by surveillance if there is complete clinical response. Dr. McKiernan started with a case study of a 70 year old male who had cT3N0M0 bladder cancer with left hydronephrosis who subsequently had a percutaneous nephrostomy tube placement.
He had a complete clinical response with neoadjuvant chemotherapy (gemcitabine/cisplatin) and resolution of hydronephrosis but ultimately refused radical cystectomy. Five years later he developed CIS and responded to induction BCG. Another 7 years later he developed Gleason Group 4 prostate cancer and underwent ADT + radiotherapy. Five years after the prostate cancer diagnosis, he was still NED and died of COVID pneumonia at the age of 87.
Based on 20 years of data, we know that pT0 rates are approaching 40%: 38% in the SWOG, PURE-01, and AMVAC trials,1,2, and 42% in the VESPER trial.3 The AUA Guidelines suggest that in statement 6 “utilizing a multidisciplinary approach, clinicians should offer cisplatin-based neoadjuvant chemotherapy to eligible patients prior to radical cystectomy (Strong recommendation).” Of note, the best regimen and duration for cisplatin-based neoadjuvant chemotherapy remains to be defined.
Dr. McKiernan highlighted that there are 82,000 new bladder cancer diagnoses in 2023, of which 20,000 are muscle invasive disease. We know that ~42% of patients will be pT0 with neoadjuvant chemotherapy, thus 8,400 patients have a radical cystectomy with pT0 disease. Furthermore, radical cystectomy is associated with a 2.7% mortality rate, 25% readmission rate, and 64% complication rate. This translates to: 227 deaths, 2,100 readmissions, and 5,376 complications, all from an operation on patients that don’t have cancer in the organ they were operated on. Furthermore, the therapeutic benefit of radical cystectomy in pT0 is unknown, as 6-10% will relapse systemically. Thus, it is difficult to determine pathologic complete response rates without using radical cystectomy as a “biopsy.”
The AUA guidelines based on statement 5 suggest that “clinicians should counsel patients regarding complications and implications of treatment on quality of life (ie. impact of continence, sexual function, fertility, bowel dysfunction, and metabolic problems). Indeed, high grade complications are observed in approximately 20% of patients. Moreover, mortality rates are <3% in most series, but can be as high as 4-6%. Readmission rates are typically 10-30%, and a recent review suggested that up to 26% require ICU admission post-radical cystectomy.
Based on the pT0 rate of 40%, there are several follow-up questions that remain:
- Is avoiding a radical cystectomy a desirable endpoint?
- Is there benefit in removing the bladder with no cancer?
- Can we accurately determine when cT0 = pT0 after neoadjuvant chemotherapy?
- Can delayed radical cystectomy rescue relapses on surveillance?
- Do non-muscle invasive bladder cancer recurrences following pathologic complete response pose a threat to the patient?
- Can MRI, extent of TURBT, or genomics play a role for these patients?
- Can “adjuvant” systemic therapy be applied to patients who do not undergo a radical cystectomy?
In a study from Columbia University and Memorial Sloan Kettering Cancer Center, Dr. McKiernan notes that among 148 patients who experienced a clinically complete response to neoadjuvant platinum based chemotherapy and elected active surveillance, over a median follow-up of 55 months, the 5-year disease specific, overall, cystectomy-free and recurrence-free survival rates were 90%, 86%, 76%, and 64%, respectively: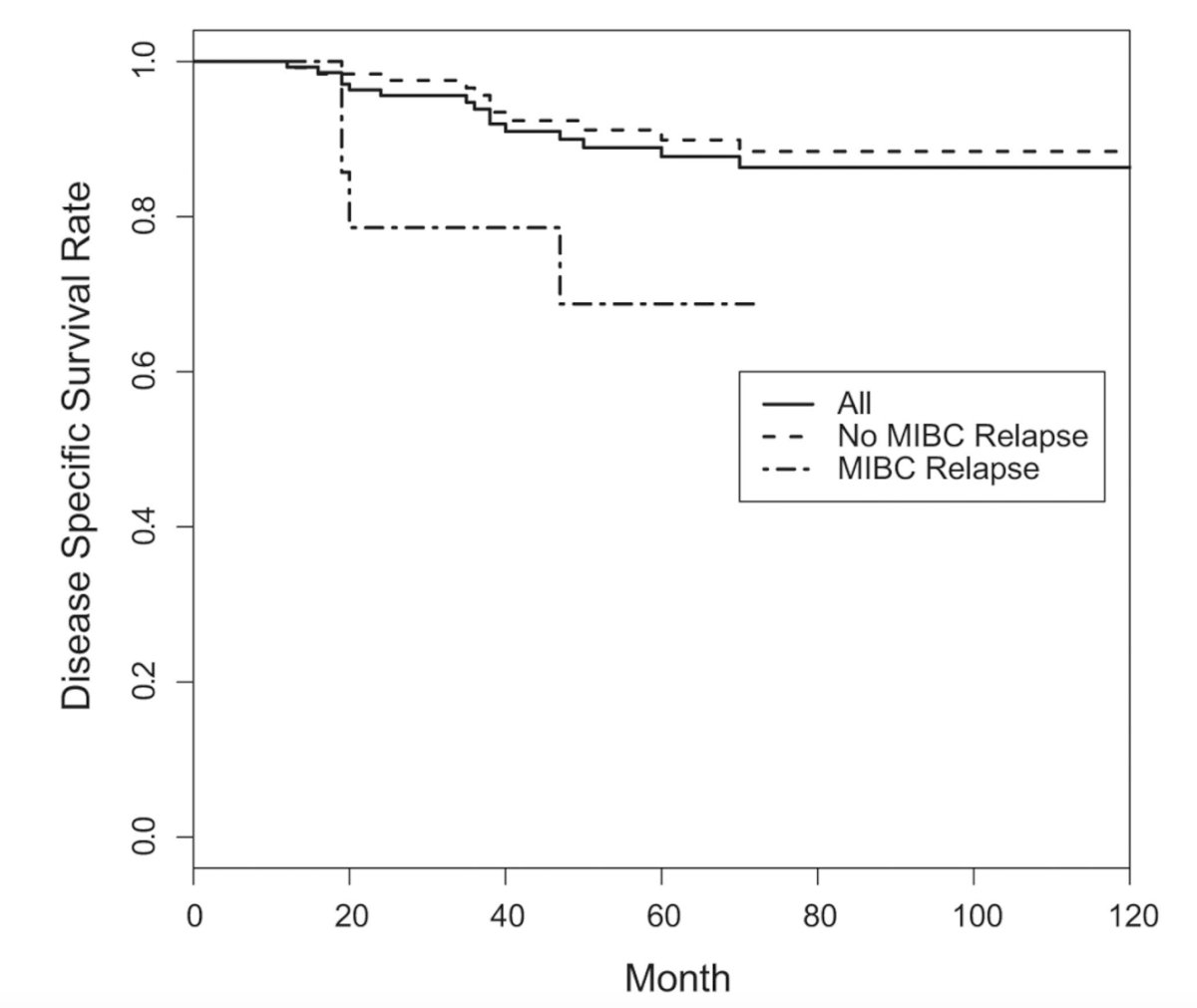
Overall, 48% of patients recurred in the bladder (11% muscle invasive disease, 37% non-muscle invasive bladder cancer), and delayed radical cystectomy prevented cancer death in 75% of patients after muscle invasive bladder cancer relapse and 93% after non-muscle invasive bladder cancer relapse. Ultimately, 3% of patients relapsed systemically with a disease free bladder. Thus, 2.7% of patients experienced an intra-bladder relapse that could not be salvaged by radical cystectomy leading to mortality.
The impact of misclassified clinical complete response carries significant ramifications. In data presented at the 2021 AUA annual meeting, 54 patients with clinical complete response were followed for 49 months. Overall, there was 11% of patients with “residual” muscle invasive bladder cancer < 6 months after pathologic complete response rate, with pre-neoadjuvant chemotherapy in these patients, being more common (67% vs 8%, p< 0.01). The 5 year overall survival was 63% versus 80% for those that achieved a durable clinical complete response (p = 0.05). Importantly, the 11% risk of misclassification was associated with a 17% decrease in overall survival. Additionally, there was a 1.9% risk of mortality secondary to surveillance in patients with complete clinical response, which is lower than the mortality rate associated with radical cystectomy at centers of excellence.
Dr. McKiernan then highlighted data presented at this meeting (SUO 2023), notably that 61 patients with clinical complete response had a 46% relapse with non-muscle invasive bladder cancer on surveillance. Among these patients, 80% were high grade and 80% were treated without radical cystectomy. Importantly, non-muscle invasive bladder cancer recurrences did not predict muscle invasive bladder cancer or metastases. The AUA guidelines, based on statement 29 suggest that “in patients who have a NMIBC recurrence after bladder preserving therapy, clinicians may offer either local measures, such as TURBT with intravesical therapy, or radical cystectomy with bilateral pelvic lymphadenectomy (Moderate recommendation)”:
Patient selection in this population is key. Data from the NCDB assessed population level data on TURBT + neoadjuvant chemotherapy from 2004-2015, of which 1,538 patients underwent TURBT + chemotherapy and no radical cystectomy. Overall, stage, race, insurance, and facility type predicted absence of radical cystectomy, and 2 year survival with TURBT + chemotherapy was 49% (and 53% if cT2):4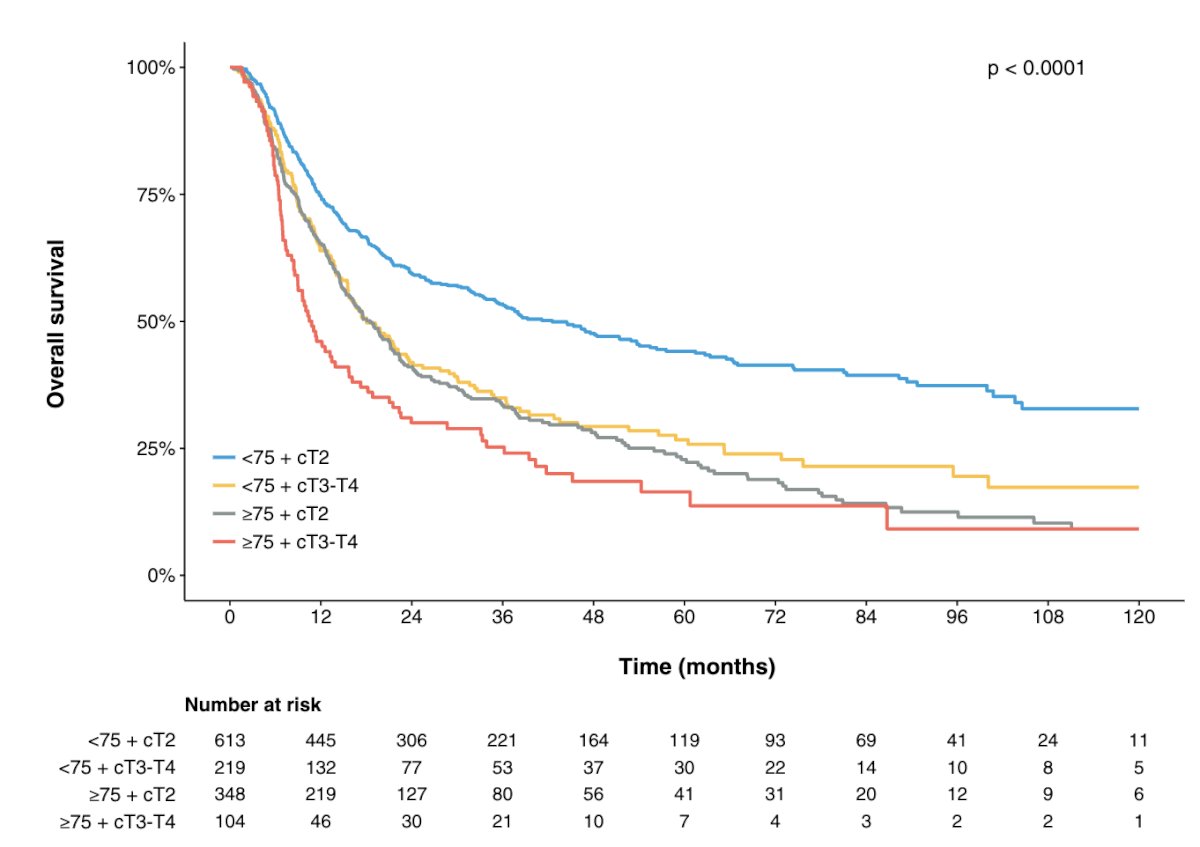
Ultimately, this study taught us that the omission of radical cystectomy in an unselected population is bad. The ideal patient factors associated with patients that will do well with bladder preservation/surveillance are as follows:
- Absence of secondary muscle invasive bladder cancer
- Absence of CIS
- Absence of variant histology
- Absence of hydronephrosis
- Multiparametric MRI findings
- Re-TURBT findings pre-neoadjuvant chemotherapy
Additionally, there are important genomic considerations, including DDR tumor mutation status and ctDNA being negative.
Dr. McKiernan then discussed the prospective trials assessing surveillance in cT0, specifically the RETAIN trial. This trial is assessing neoadjuvant aMVAC and molecular profiling in a risk-adapted approach with the following trial design:
The 2-year bladder intact with no metastasis rate was 46%, and the 2-year metastasis free survival was 77% in active surveillance and 71% in the radical cystectomy group (p = 0.82). The 2-year overall survival rate was 84% and 89% in the radical cystectomy and active surveillance group, respectively.
Another trial in this disease space is the Alliance A031701 phase II trial, which has accrued 172 patients of which 24% have a DDR gene alteration. Of note, this trial allows for residual CIS and BCG, radiotherapy, or radical cystectomy as definitive therapy. The trial schema for this trial is as follows: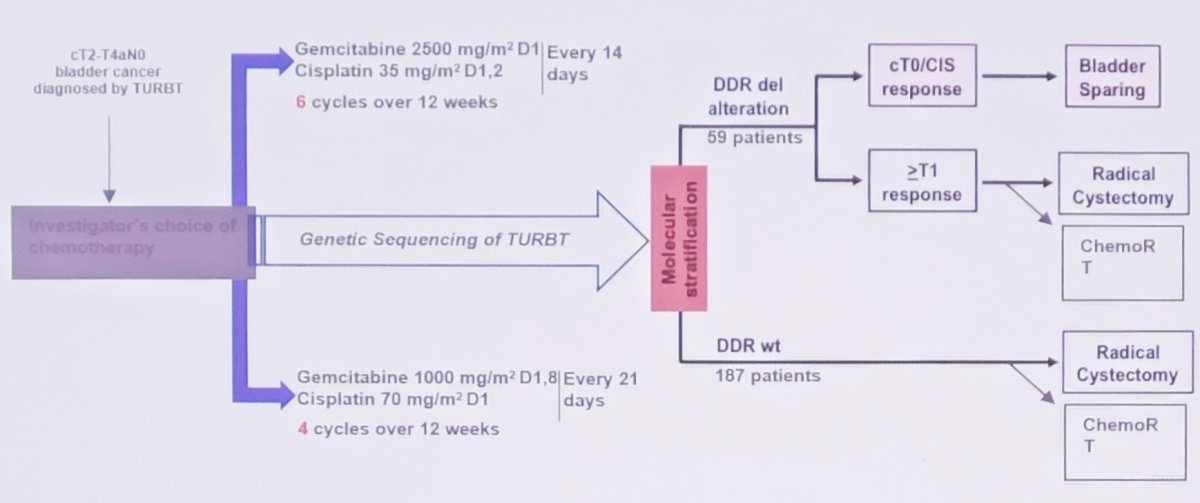
So, for complete clinical response, what do the guidelines say?
- AUA
- Clinicians should perform a radical cystectomy as soon as possible following neoadjuvant chemotherapy (ideally within 12 weeks)
- For patients with newly diagnosed muscle invasive bladder cancer who desire to retain their bladder, and for those with significant comorbidities, clinicians should offer bladder preserving therapy when it is clinically appropriate
- EAU
- Overtreatment is a possible negative consequence of radical cystectomy
- Neoadjuvant chemotherapy alone rarely produces durable complete response despite clinical complete response up to 56%
- Bladder-conserving strategies with TUR and systemic chemotherapy could lead to long-term survival with intact bladders in selected patients
There are several future directions that will push the concept to be safer according to Dr. McKiernan:
- Multiple TURs: TUR, TUR, re-TUR
- mpMRI and VI-RADS for clinical staging
- ctDNA
- Improvements in systemic therapy
Again, referencing the guidelines, Dr. McKiernan notes that with regards TUR, there are two important statements:
- Statement 22: “In patients under consideration for bladder preserving therapy, maximal debulking TURBT and assessment of multifocal disease/CIS should be performed (Strong recommendation)
- Statement 25: “For patients with muscle invasive bladder cancer who have elected multi-modal bladder preserving therapy, clinicians should offer maximal TURBT, chemotherapy combined with external beam radiation therapy, and planned cystoscopic re-evaluation (Strong recommendation).
Work from Dr. McKiernan’s group suggests that TUR can play a role in pT0 rates.5 Among 93 patients, 62 (67%) underwent complete TURBT prior to chemotherapy. Compared to patients with incomplete TURBT, those with complete TURBT had lower rates of variant histology (13% vs. 32%) and hydronephrosis (15% vs. 39%). Patients with complete TURBT were more likely to defer radical cystectomy and pursue active surveillance (61% vs. 32%). Patients with complete TURBT had higher 5-year overall (77% vs. 46%, p = 0.003) and cancer-specific (85% vs. 50%, p = 0.001) survival: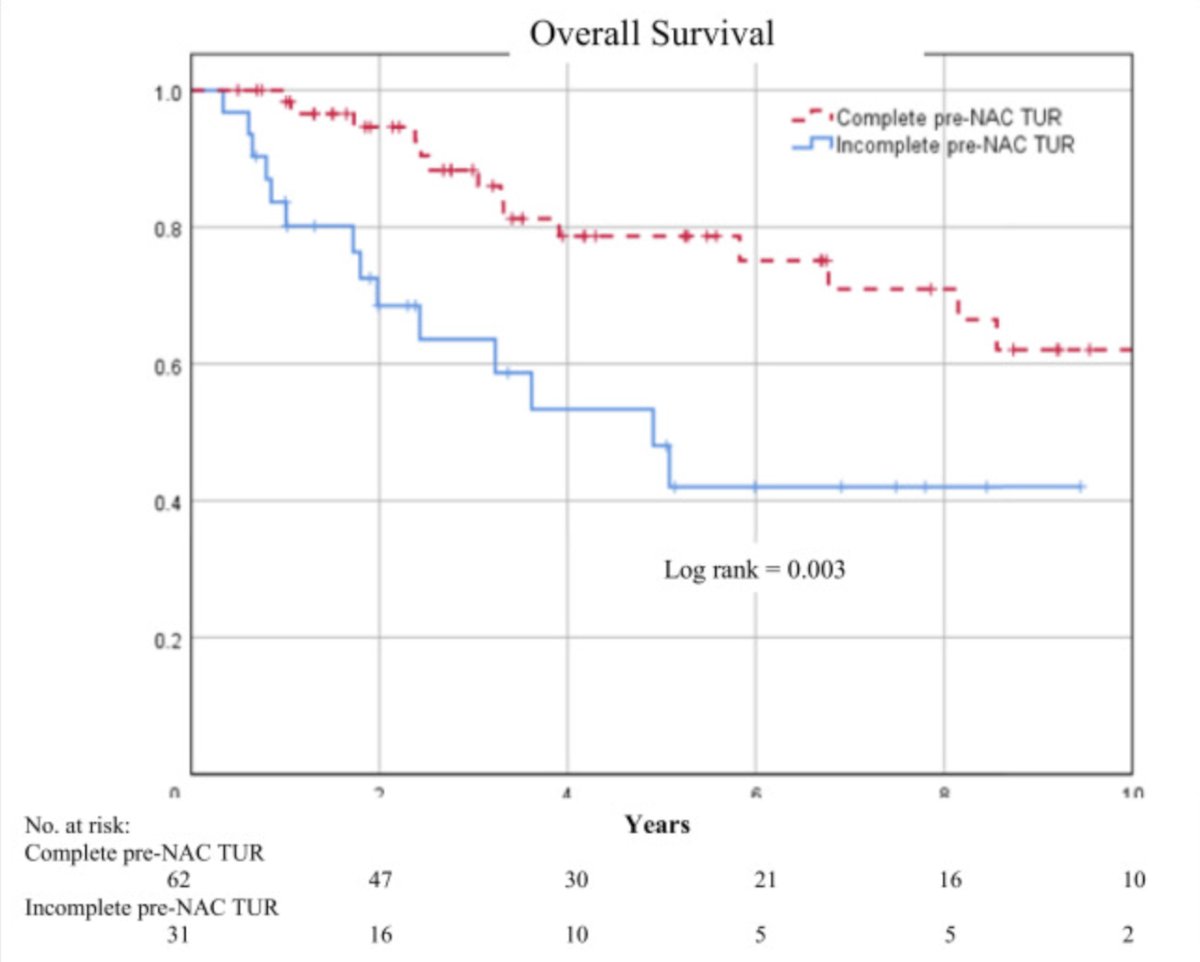
The role of ctDNA in this disease space has been highlighted in the ABACUS trial, suggesting that ctDNA status is highly prognostic, with no relapses in ctDNA-negative patients at baseline and after neoadjuvant therapy.6 Indeed, work presented at ASCO 2023 suggests that ctDNA status predicts pathological complete response. Among 68 patients with muscle invasive bladder cancer that had neoadjuvant chemotherapy + radical cystectomy, ctDNA was measured at baseline and prior to radical cystectomy. There were 59% of patients that were ctDNA negative pre-neoadjuvant chemotherapy, with 84% having a pathologic complete response versus 41% that were ctDNA positive, with 35% having a pathologic complete response. Post neoadjuvant chemotherapy, 84% were ctDNA negative with 81% of patients having pathologic complete response versus no patients that were ctDNA positive post neoadjuvant chemotherapy achieving a pathologic complete response. Thus, ctDNA status at both time points predicted pathologic complete response, and ctDNA status was a better predictor of RFS than pathologic complete response. Ultimately, there should be no surveillance for ctDNA positive patients after neoadjuvant chemotherapy regardless of clinical complete response.
Based on data from CheckMate 2747 and Javelin Bladder 100,8 Dr. McKiernan suggests that it is likely that “adjuvant” systemic therapy and “maintenance” therapy, respectively, does not require radical cystectomy in order to have efficacy (but, there are no trials assessing this directly).
Dr. James McKiernan concluded his presentation discussing neoadjuvant chemotherapy followed by surveillance if there is complete clinical response in the setting of muscle invasive bladder cancer with the following take-home points:
- Surgical consolidation for pT0 is prophylactic at best
- Identifying cT0 = pT0 without radical cystectomy is a challenge
- Risk of misclassification results in a mortality risk
- Risk of non-muscle invasive bladder cancer recurrence is high and impact on overall survival is low
- Patient selection is key
- Maximal TUR, mpMRI, and ctDNA will improve patient selection
- Improvements in systemic therapy will continue to push us to debate this topic
Presented by: James McKiernan, MD, Columbia University, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 Society of Urologic Oncology (SUO) Annual Meeting, Washington, D.C., Tues, Nov 28 – Fri, Dec 1, 2023.
References:
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349(9):859-866.
- Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients with Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J Clin Oncol 2018 Dec 1;36(34):3353-3360.
- Pfister C, Gravis G, Flechon A, et al. Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine and cisplatin as perioperative chemotherapy for patients with nonmetastatic muscle-invasive bladder cancer: Results of the GETUG-AFU V05 VESPER trial. J Clin Oncol. 2022 Mar 7 [Epub ahead of print].
- Audenet F, Waingankar N, Ferket BS, et al. Effectiveness of transurethral resection plus systemic chemotherapy as definitive treatment for muscle invasive bladder cancer in population level data. J Urol. 2018 Nov;200(5):996-1004.
- Pak JS, Haas CR, Anderson CB, et al. Survival and oncologic outcomes of complete transurethral resection of bladder tumor prior to neoadjuvant chemotherapy for muscle-invasive bladder cancer. Urol Oncol. 2021 Nov;39(11):787.e9-787.e15.
- Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. 2019 Nov;25(11):1706-1714.
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021 Jun 3;384(22):2102-2114.
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020 Sept 24;383(13):1218-1230.


