(UroToday.com) The 2023 Society of Urologic Oncology (SUO) annual meeting held in Washington, D.C. between November 28th and December 1st, 2023, was host to a poster/abstract session. Dr. Stephen Boorjian presented the 36 months follow-up results of the phase 3 trial evaluating the efficacy of intravesical nadofaragene firadenovec-vcng for patients with Bacillus Calmette-Guerin (BCG)-unresponsive carcinoma in situ (CIS) of the bladder.
Patients with BCG-unresponsive non-muscle invasive bladder cancer (NIMBC) remain at significant risk for disease recurrence and, more importantly, progression. Radical cystectomy remains the guideline-recommended, gold standard treatment approach for such patients. Along with Keytruda® (pembrolizumab), nadofaragene firadenovec (Adstiladrin®) is United States Food and Drug Administration (FDA)-approved for the treatment of BCG-unresponsive NMIBC. Nadofaragene firadenovec-vncg is a non-replicating adenoviral vector-based gene therapy that delivers human interferon alpha-2b to urothelial cells.
In 2021, Boorjian et al. published the primary efficacy, safety, and durability outcomes of the phase 3 trial of nadofaragene firadenovec in patients with BCG-unresponsive NMIBC.1 53.4% of patients with CIS +/- Ta/T1 papillary disease had a complete response within 3 months of the first dose, and this response was maintained in 46% of patients at 12 months. In the safety population, which included patients with both CIS +/- papillary disease and high-grade Ta/T1 disease, for most patients (66%), the study drug-related adverse events were transient and classified as either grade 1 or 2, with only 4% of patients reporting grade 3 adverse events. No grade 4 or 5 study drug-related adverse events and no treatment-related deaths were reported. The objective of this study was to report the 36-months follow-up results, including high-grade recurrence-free survival, cystectomy-free survival, and overall survival in the CIS +/- Ta/T1 cohort.
This was a single arm, open-label, multicenter phase 3 trial that enrolled 107 patients with BCG-unresponsive NMIBC with CIS +/- Ta/T1 disease. The efficacy analysis for this cohort included 103 patients who met the protocol definition of BCG-unresponsive NMIBC. Patients received 75 ml of nadofaragene firadenovec (3 x 1011 viral particles/mL) once every three months for up to four doses. Per protocol, a 5-site biopsy (dome, trigone, right and left lateral walls, and posterior wall) was performed at 12 months, and patients who were high-grade recurrence-free were offered continued treatment at the investigator’s discretion. Assessments beyond 24 months were performed in accordance with usual clinical practice. This trial remains ongoing, with a planned 5-year treatment and monitoring phase; the follow-up results presented in this report were based on the 36-months interim data for the CIS +/- Ta/T1 cohort.
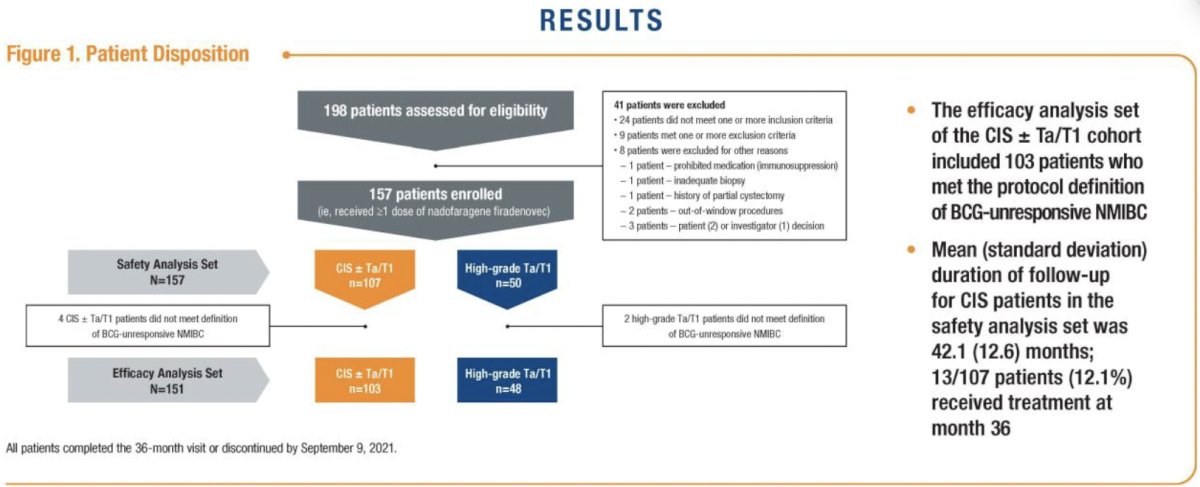
The baseline patient characteristics are summarized below. The median age was 72 years, with 89% male. The median time from initial diagnosis to treatment was 20 months. 99% of patients had received at least two prior BCG courses. CIS-only disease was present in 76% of patients.

In the overall cohort (n=103), the Kaplan-Meier estimated median duration of high-grade recurrence free-survival was 6 months (95% CI: 3.4 to 8.3 months). At 36 months, 14/55 patients (26%) who had achieved a complete response at 3 months remained free of high-grade recurrence. Four patients (4%) experienced progression to muscle-invasive disease.
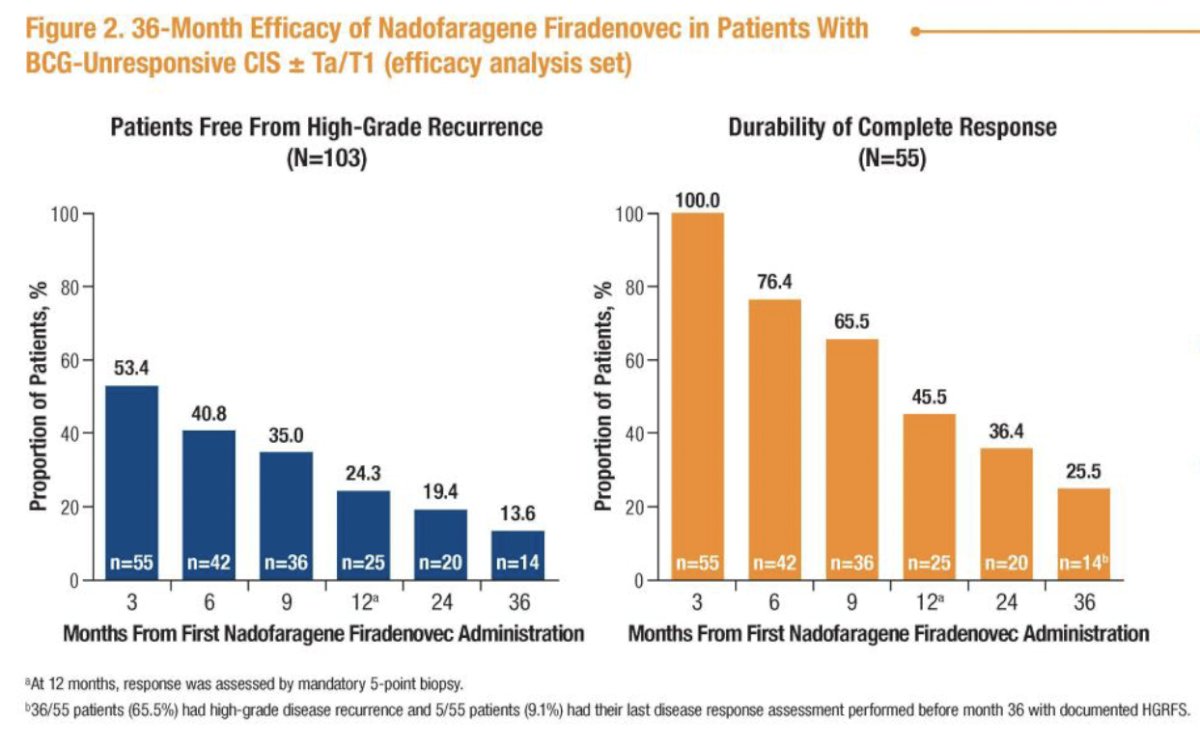
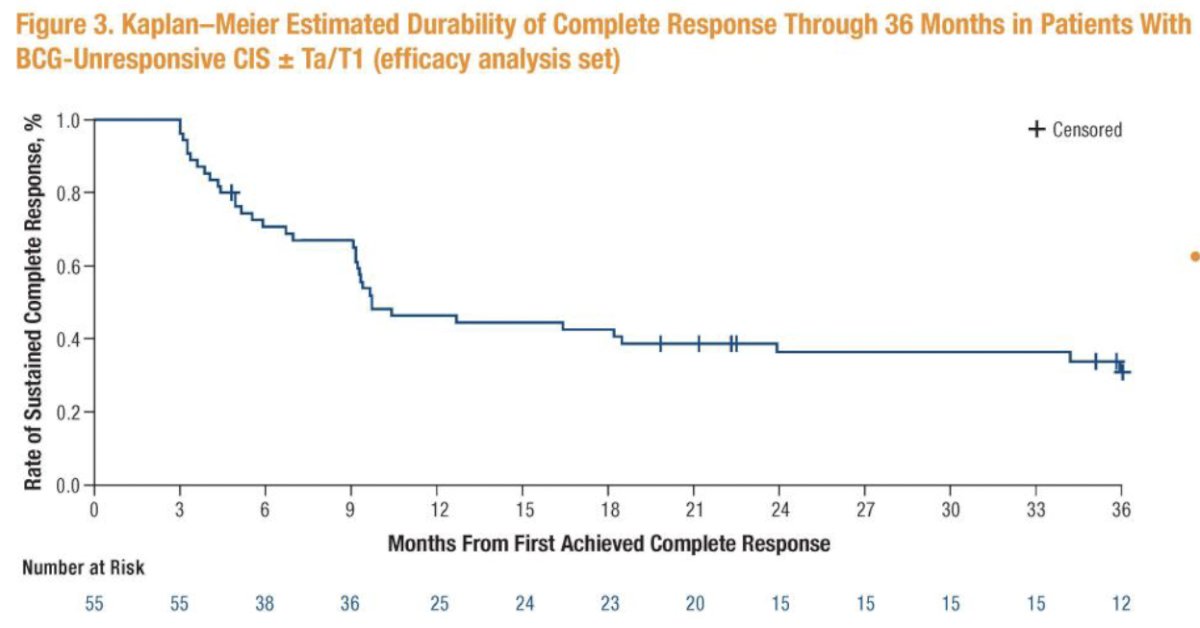
The median duration of complete response was 9.7 months (95% CI: 9.2 to 24 months), and the probability of maintaining a complete response for at least 36 months was 34.2% (95% CI: 21.6 to 47.1 months).
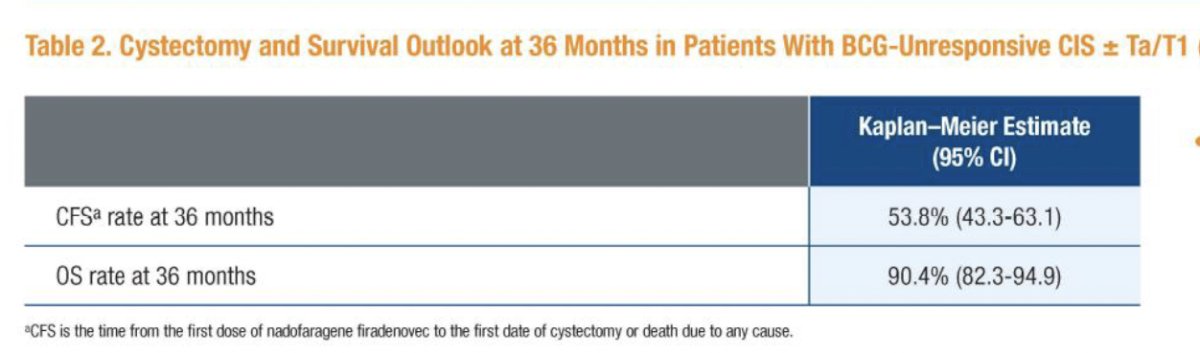
The 36-months cystectomy-free survival rate was 53.8% (95%CI: 43.3% to 63.1%), and the 3-year overall survival rate was 90.4% (95% CI: 82.3% to 94.9%).
Two patients (2%) discontinued treatment secondary to adverse events (1 patient due to grade 3 bladder spasm and another due to grade 2 instillation site discharge; neither event was considered a serious adverse event).
Dr. Boorjian and colleagues concluded with the following key takeaways:
- Intravesical nadofaragene firadenovec, administered once every 3 months, demonstrated a sustained durability of initial complete response in approximately 25% of patients with BCG-unresponsive CIS +/- Ta/T1 papillary disease through 36 months
- Nadofaragene firadenovec represents a novel treatment option for BCG-unresponsive NMIBC that advances the current treatment paradigm
Presented by: Stephen Boorjian, MD, Carl Rosen Professor and Chair of the Department of Urology, Mayo Clinic, Rochester, MN
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Society of Urologic Oncology (SUO) Annual Meeting, Washington, D.C., Tues, Nov 28 – Fri, Dec 1, 2023.
References:


