(UroToday.com) The 2023 Society of Urologic Oncology (SUO) annual meeting held in Washington, D.C. between November 28th and December 1st, 2023, was host to a poster/abstract session. Professor James Catto presented the updated safety and efficacy outcomes of THOR-2 Cohort 2 which is evaluating oral erdafitinib in patients with Bacillus Calmette-Guerin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC) and FGFR3/2 alteration.
Patients with BCG-unresponsive, high-risk NMIBC, particularly those presenting with carcinoma in situ (CIS), harbor a high risk of disease recurrence and progression. Radical cystectomy remains the guideline-recommended, gold standard treatment for these patients. However, many patients are either unfit for or refuse radical cystectomy. FDA-approved treatment options for these patients remain limited and include:
- Keytruda® (pembrolizumab) approved in January 20201
- Adstiladrin® (nadofaragene firadenovec) approved in December 20222
Fibroblast Growth Factor Receptor (FGFR) alterations may function as oncogenic drivers in NMIBC. While the frequency of such mutations is 50 to 80% in the overall NMIBC population,3 limited information exists regarding the prevalence of FGFR alterations in patients with CIS. Erdafitinib is an oral selective pan-FGFR tyrosine kinase inhibitor which is approved for adult patients with locally advanced or metastatic urothelial cancer and FGFR3/2 alterations who have progressed during or following ≥1 line of platinum-containing chemotherapy.4 Oral erdafitinib has demonstrated clinical activity in patients with high-risk NMIBC.5
THOR-2 (NCT04172675) is a multicohort phase 2 study of erdafitinib in patients with high-risk NMIBC. Cohort 2 is an exploratory cohort of patients with BCG-unresponsive CIS who harbor FGFR alterations, with or without papillary disease.
The study design is summarized below:
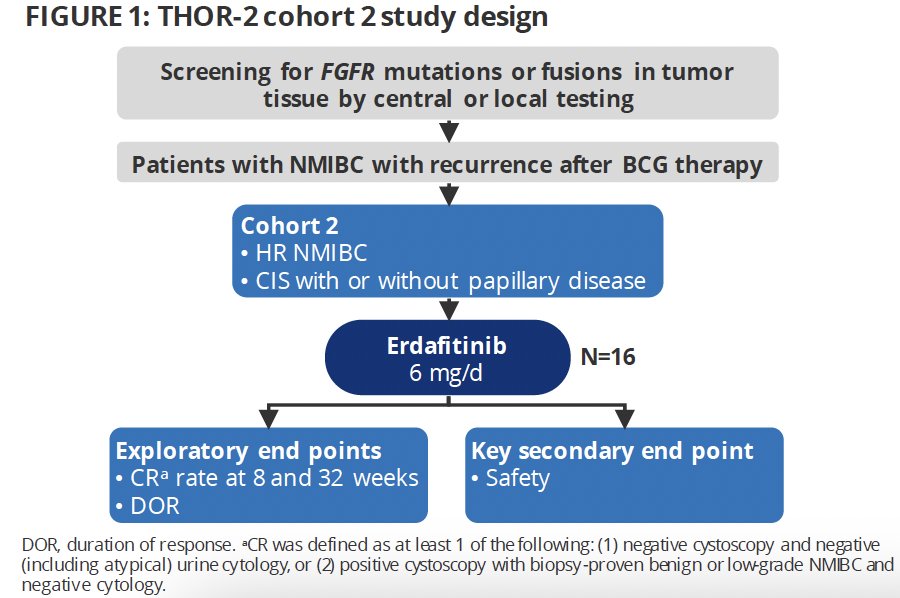
The trial eligibility criteria were as follows:
- Adult patients aged ≥18 years
- Histologically confirmed, BCG-unresponsive high-risk NMIBC
- BCG-unresponsive was defined as having persistent/recurrent CIS alone or with recurrent Ta/T1 or recurrent high-grade Ta/T1 disease ≤6 months after adequate BCG therapy, or high-grade T1 at the first disease assessment after BCG therapy
- FGFR3/2 alterations confirmed via local/central testing
- CIS +/- papillary disease
- Refusing or ineligible for radical cystectomy
Patients received oral erdafitinib 6 mg once daily without uptitration in 28-day cycles (dose selected to improve tolerability). Erdafitinib was discontinued if no complete response was observed within 3 months.
As of the data cut-off of June 27, 202), 16 patients had received erdafitinib. The median study follow-up was 14.8 months, and the median duration of treatment was 6.7 months (range: 1.9 - 18.6). The median age was 68.5 years (range: 35 - 83). Consistent with the study eligibility criteria, all patients had CIS, with >90% having an FGFR3 mutation.
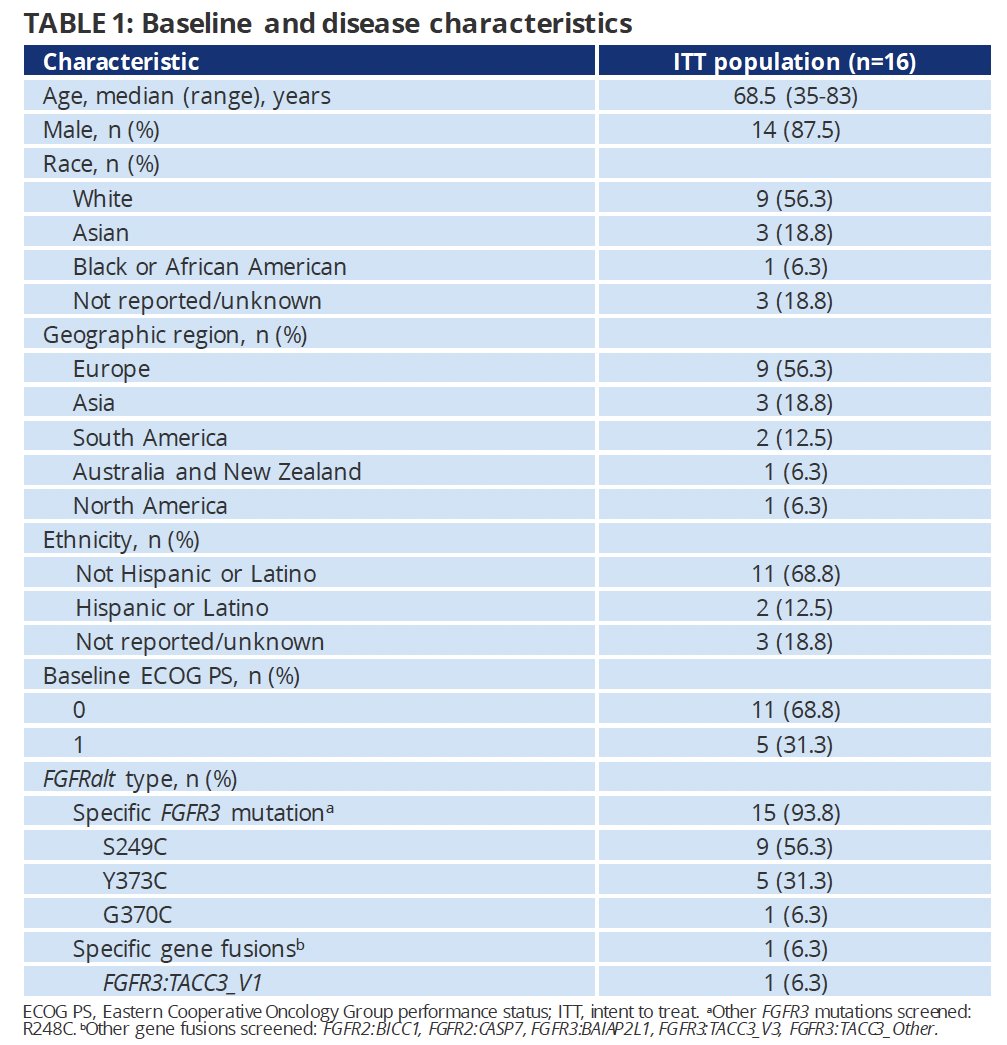
From an efficacy standpoint, the complete response rate in disease-evaluable patients was 94% (15/16) at 8 weeks and 73% (8/11) at 32 weeks.
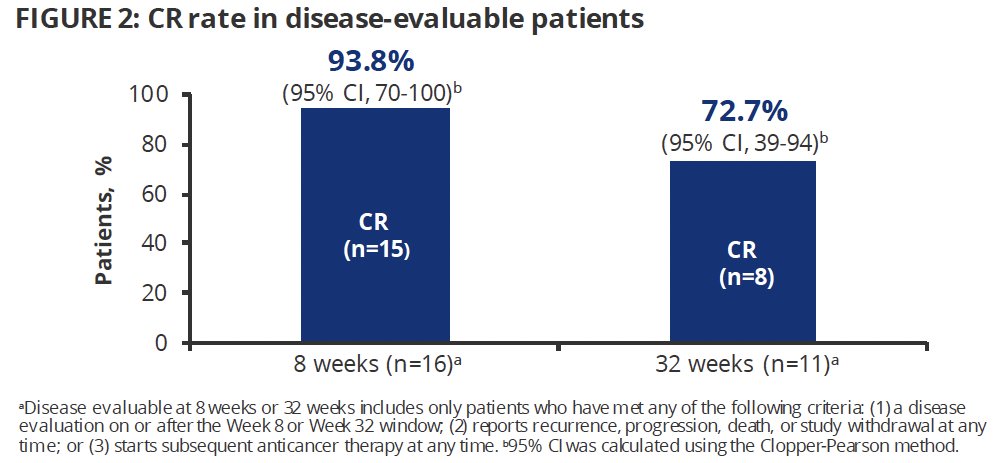
The median duration of response was not reached; 12 patient responses were ongoing at data cut-off.
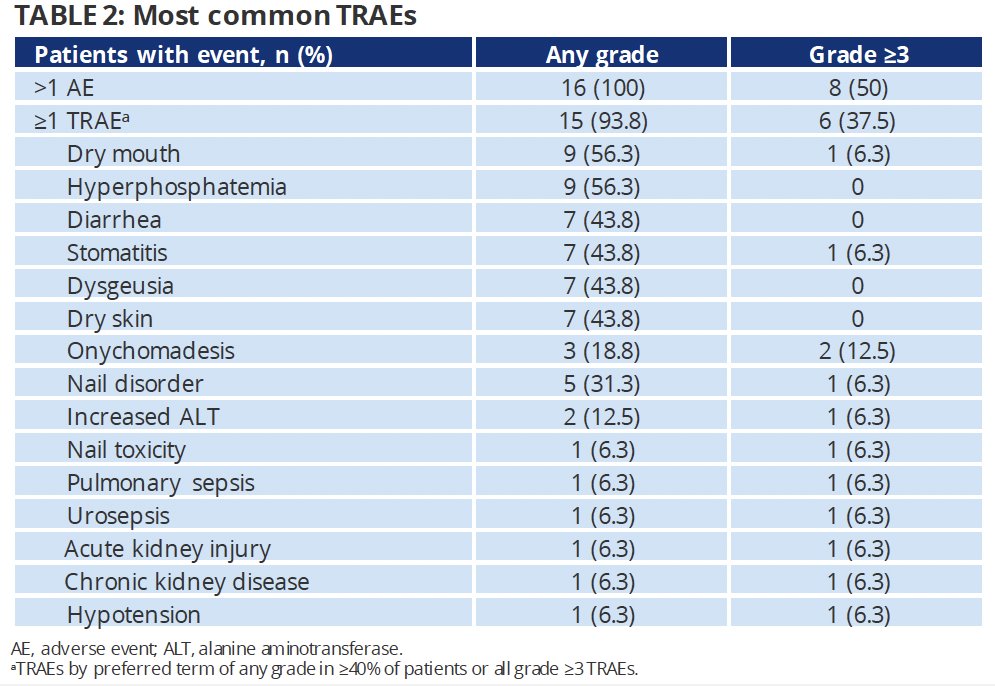
Any grade treatment-related adverse events (TRAEs) were observed in 94% of patients. The most common TRAEs were dry mouth, hyperphosphatemia (56.3% each), diarrhea, stomatitis, dysgeusia, and dry skin (44% each). Grade 3+ TRAEs occurred in 6 (38%) patients, with the most common being onychomadesis (13%) or nail disorders (6.3%).
Professor Catto concluded with the following key takeaways and conclusions:
- In cohort 2 of THOR-2, erdafitinib demonstrated promising and durable efficacy in patients with BCG-unresponsive high-risk CIS +/- papillary disease and FGFR alterations
- The complete response rates after 8 and 32 weeks were 94% and 73%, respectively
- The median duration of response was not reached, and 12 responses were still ongoing at the data cut-off
- Notably, the 2 longest response durations were observed in patients who discontinued treatment relatively early, highlighting the durability of response off treatment.
- The safety data were consistent with the known safety profile of erdafitinib
- Most TRAEs were grade 1 or 2; one patient discontinued erdafitinib because of a TRAE
- TAR-210, a novel intravesical drug delivery system that provides sustained, local release of erdafitinib within the bladder while limiting systemic exposure, is currently being evaluated in an ongoing trial (NCT05316155)
Presented by: Professor James Catto, MBChB, PhD, FRCS, Professor of Urologic Surgery at the University of Sheffield, Sheffield, United Kingdom
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Society of Urologic Oncology (SUO) Annual Meeting, Washington, D.C., Tues, Nov 28 – Fri, Dec 1, 2023.
References:
- FDA approves pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive bladder cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer. Accessed on November 30, 2023.
- FDA D.I.S.C.O. Burst Edition: FDA approval of Adstiladrin (nadofaragene firadenovec-vncg) for patients with high-risk Bacillus Calmette-Guérin unresponsive non-muscle invasive bladder cancer with carcinoma in situ with or without papillary tumors. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-adstiladrin-nadofaragene-firadenovec-vncg-patients-high-risk#:~:text=On%20December%2016%2C%202022%2C%20the,with%20or%20without%20papillary%20tumors. Access on November 30, 2023.
- Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015;15(1):25-41.
- Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2019;381:338-348.
- Catto JWF, Tran B, Roupret M, et al. Erdafitinib in BCG-treated high-risk non-muscle-invasive bladder cancer. Ann Oncol. 2023; S0923-7534(23)04015-2.


