(UroToday.com) The 2023 Society of Urologic Oncology (SUO) annual meeting held in Washington, D.C. between November 28th and December 1st, 2023, was host to a poster/abstract session. Dr. Siamak Daneshmand presented the updated Cohort 3 results from THOR-2, a marker lesion study of oral erdafitinib in patients with intermediate-risk, non-muscle invasive bladder cancer (NMIBC).
Current treatments for papillary NMIBC involve adjuvant therapy that precludes on-target efficacy assessment of measurable disease. Measurable disease by marker lesions provides an opportunity to assess the on-target efficacy of novel therapies. Fibroblast Growth Factor Receptor (FGFR) alterations occur in 50 to 80% of NMIBC patients and such mutations may function as oncogenic drivers.1 Erdafitinib is an oral selective pan-FGFR tyrosine kinase inhibitor that is approved for adult patients with locally advanced or metastatic urothelial cancer and FGFR3/2 alterations who have progressed during or following ≥1 line of platinum-containing chemotherapy.2 Oral erdafitinib has demonstrated clinical activity in patients with high-risk NMIBC.3
THOR-2 (NCT04172675) is a multicohort phase 2 study of erdafitinib in patients with high-risk NMIBC. Cohort 3 is an exploratory marker lesion cohort of patients with low-grade, intermediate-risk NMIBC. The study design is as follows:
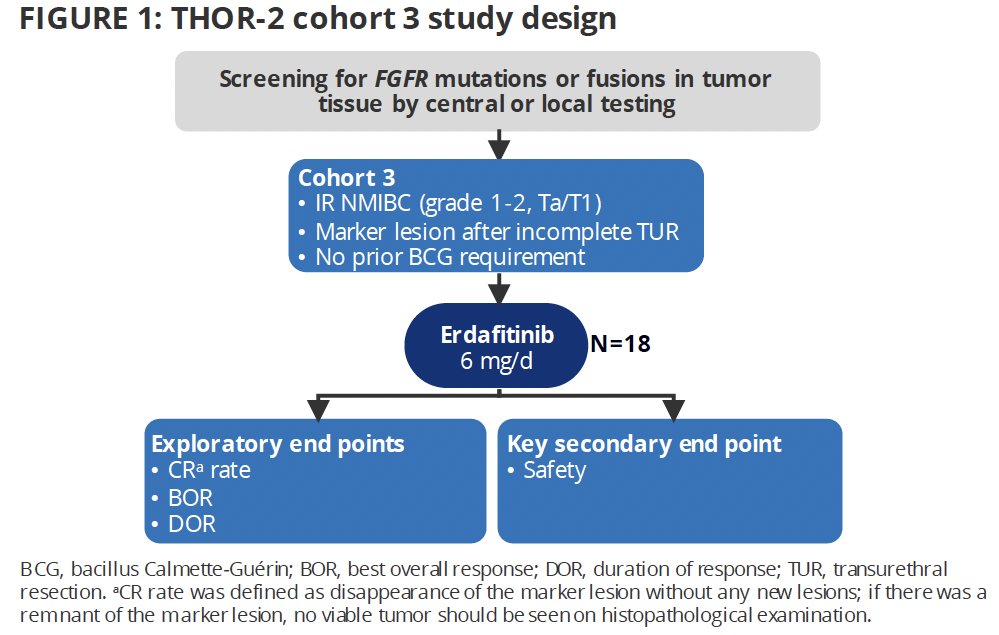
THOR-2 Cohort 3 included patients meeting the following eligibility criteria:
- Adult patients aged ≥18 years
- Histologically confirmed recurrent grade 1-2 Ta/T1 intermediate risk NMIBC with FGFR3/2 alterations
- No previous carcinoma in situ (CIS)
- Risk of progression <5% in the next 2 years and >50% risk of recurrence using the European Organisation for Research and Treatment of Cancer (EORTC) risk calculator
Patients had all visible bladder tumors removed by resection or fulguration except for one 5 to 10 mm bladder tumor (marker lesion). The patients received oral erdafitinib 6 mg once daily without uptitration in 28-day cycles. Cystoscopy was done on day 1 of cycles 2, 3, and 4 or until complete response, if earlier, and if a complete response was achieved, urine cytology was performed. Patients with a partial response partial or complete response ≤3 months after starting treatment continued erdafitinib for ≤2 years or until the development of high-risk disease recurrence, intolerable toxicity, consent withdrawal, investigator decision, or study closure.
As of the data cut-off of June 27, 2023, 18 patients had received erdafitinib. The median study follow-up was 10 months, and the median treatment duration was 7.1 months (range: 0.7 – 17.4 months). The median patient age was 63.5 years (range: 47 – 77). Of the 18 patients, 13 had tumor stage Ta (remaining five had no tumor stage reported). Consistent with the eligibility criteria, all patients had FGFR3 mutations.
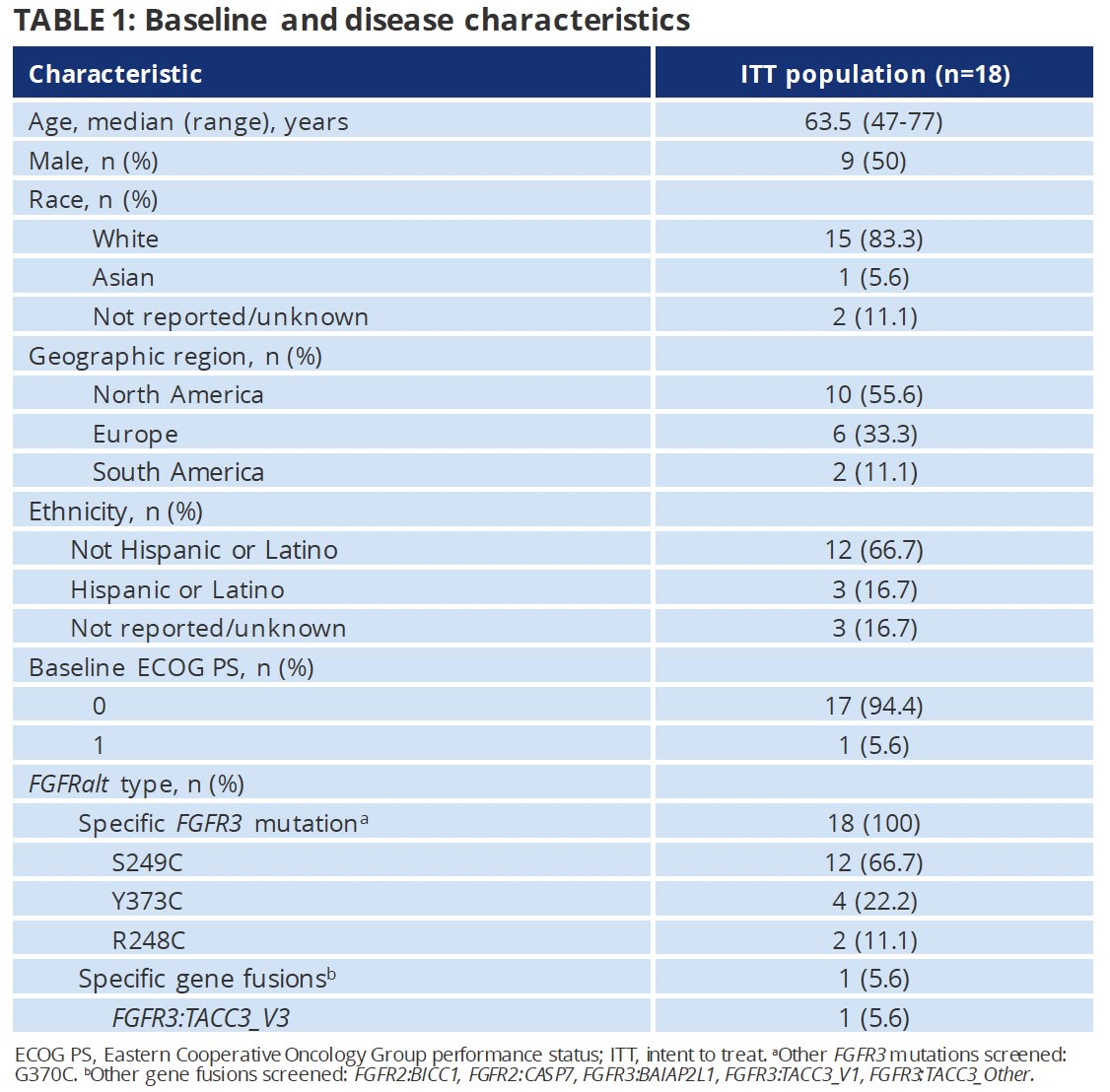
From an efficacy standpoint, 15/18 (83%) patients had a complete response, two (11%) had a partial response, and one (5.6%) had high-grade recurrence as the best objective response.
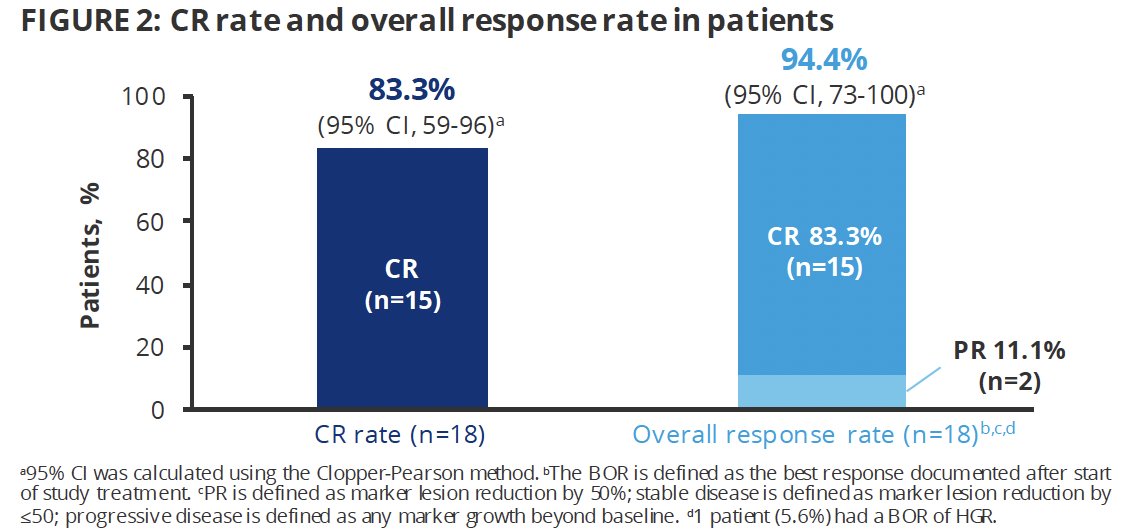
The median time to response was 1.15 months. Among the 17 responders, the median duration of response was 12.7 months, with 12 patient response ongoing, three censored, and two ending with recurrence (1 low grade and high grade each) at the data cut-off.
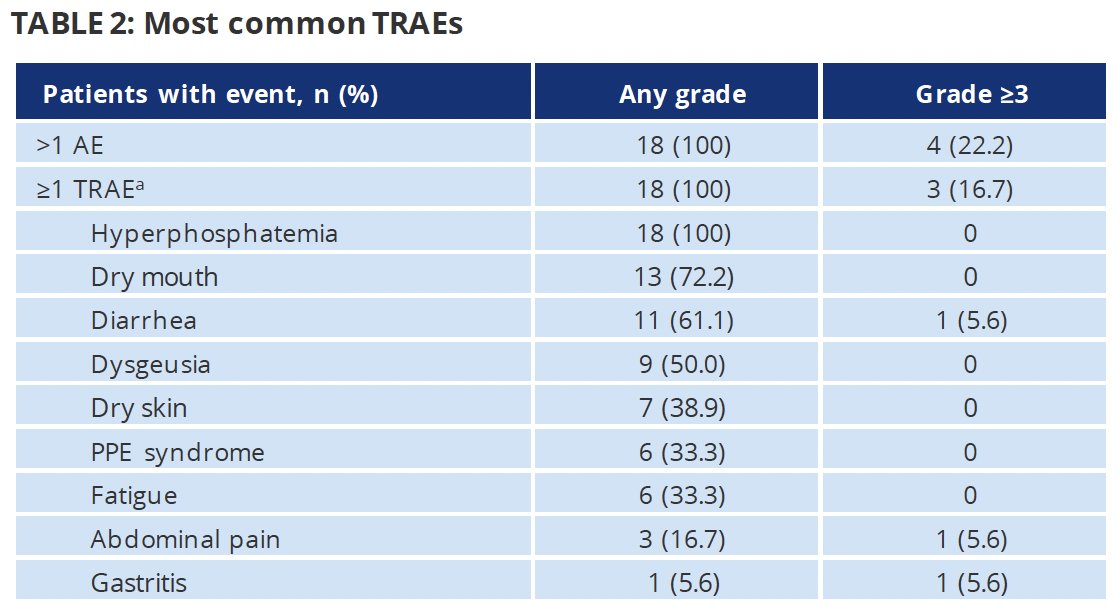
Any grade treatment-related adverse events (TRAEs) occurred in all patients. The most common TRAEs were hyperphosphatemia (100%), dry mouth (72%) and diarrhea (61%). Grade 3+ TRAEs occurred in 17% and included: abdominal pain, gastritis, and diarrhea (5.6% each). Three patients discontinued erdafitinib secondary to TRAEs
Dr. Daneshmand concluded with the following key takeaways messages:
- In cohort 3, a marker lesion study of THOR-2, erdafitinib demonstrated promising anti-tumor activity in adult patients with intermediate-risk NMIBC and FGFR alterations
- The complete response rate was 83%, with a median duration of response of 12.7 months, with 12 responses still ongoing at data cut-off
- Data from this trial demonstrates the rapid onset and durability of responses following treatment with erdafitinib in patients with intermediate-risk NMIBC
- Among responders, the median duration of response was just over one year
- Safety data were consistent with the known safety profile of erdafitinib
- Most TRAEs were grade 1 or 2; three patients discontinued erdafitinib secondary to TRAEs
- To potentially reduce systemic toxicities in NMIBC, TAR-210, a novel intravesical drug delivery system that provides sustained, local release of erdafitinib within the bladder while limiting systemic exposure, is being evaluated in an ongoing clinical trial (NCT05316155)
Presented by: Siamak Daneshmand, MD, Professor of Urology, Department of Urology, University of Southern California, Los Angeles, CA
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Society of Urologic Oncology (SUO) Annual Meeting, Washington, D.C., Tues, Nov 28 – Fri, Dec 1, 2023.
References:
- Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015;15(1):25-41.
- Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2019;381:338-348.
- Catto JWF, Tran B, Roupret M, et al. Erdafitinib in BCG-treated high-risk non-muscle-invasive bladder cancer. Ann Oncol. 2023; S0923-7534(23)04015-2.


