(UroToday.com) The 2023 SUO annual meeting included a state of the art lecture by Dr. Ariana Smith discussing gynecologic considerations for the urologic oncologist and optimizing care for women. Dr. Smith noted that the main learning objectives for her talk are to emphasize assessment of the cervix, assessment of the uterus, and management of the adnexa.
One common myth is that the gynecologic organs have no benefit to women after menopause, however, the truth is that these organs are associated with ongoing hormone production, there is benefit in ovarian sparing, and there may be a detrimental psychosocial impact if these organs are removed. As such, the optimization of surgical care for women is multifactorial, including gynecologic history, loss of femininity, hormonal effects, sexual dysfunction, and risk of cancer:
Specific to the gynecologic organs, the goals of surgery are (i) oncologic/anatomic success, (ii) avoidance of subsequent surgery, (iii) urinary function/continence, and (iv) bowel and sexual function. Dr. Smith notes that several important gynecologic evaluations should be undertaken prior to urologic surgery, including screening for cervical pathology (PAP and HPV testing), assessing for occult uterine malignancies, considerations for managing the adnexa (+/- oophorectomy and salpingectomy), and assessing degree of pelvic support to minimize risk of pelvic organ prolapse post-surgery:
Although the incidence of occult gynecologic malignancy is low (uterine cancer – 0.7%; cervical cancer – 0%; ovarian cancer 0%), these screening considerations should be on the evaluation checklist for all urologic oncologists. Ultimately, there are three important questions to answer:
- Do I need to remove the uterus and cervix?
- What is my patient’s risk of occult uterine malignancy?
- How should I manage the adnexa?
A female cystectomy standard of care is removal of the bladder, uterus, ovaries, and fallopian tubes:
Why is this standard of care? It avoids a need for reoperation and simpler, but Dr. Smith emphasizes again that the risk of gynecologic malignancy is low. Perhaps it is time to challenge the standard of care. Dr. Smith’s group assessed 123 women with a median age of 71 years who underwent radical cystectomy with removal of reproductive organs for primary bladder cancer. Of note, 19 women (15%) had reproductive organ involvement by bladder cancer, and 5 of them (4%) were specifically found to have ovarian involvement. No women in this cohort had a primary ovarian malignancy detected at the time of radical cystectomy.
With regards to cervical screening, the American College of Obstetricians and Gynecologists’ 2016 guidelines in women > 30 years of age should preferably have HPV and cytology every five years, although acceptable is cytology every 3 years. There is no recommendation for screening after the age of 65. High risk HPV is considered HPV16, HPV18, and HPV31. The cytologic classification of squamous cell abnormalities is as follows:
Additionally, the cytologic classification of glandular cell abnormalities is as follows:
If the cervical screening necessitates a cervical biopsy, if it is positive, it will be either (i) cervical intraepithelial neoplasia (CIN) 1, 2 or 3, (ii) adenocarcinoma in situ, or (iii) invasive malignancy. In these situations, surgical options include total hysterectomy, uterine sparing, or treatment of the dysplasia. For women with abnormal screening and a negative cervical biopsy, the risk of high grade CIN is still 5.5% - 24%, whereas after a negative PAP/HPV the risk of high grade CIN is <3%. Among women previously treated for cervical dysplasia, the 5-year risk of high grade CIN or worse is 5% - 16%, however in women with two negative PAPs + HPV testing following treatment, the risk of high grade dysplasia is only 1.5%.
For patients who are post-cystectomy and requiring cervical screening, there are two categories of patients:
- Those that underwent uterine sparing: screening until they are older than 65 years of age and beyond 65 years of age if there is a history of cervical dysplasia
- Those that underwent a total hysterectomy: no need for uterine screening, unless there is a history of cervical dysplasia, then they should have ongoing vaginal PAP screening
Dr. Smith then discussed occult uterine cancer, for which there is a 1-2.7% lifetime risk of endometrial cancer or sarcoma:
When patients have vaginal bleeding, the first consideration for endometrial cancer screening is whether they are pre-menopausal or post-menopausal. For women with post-menopausal bleeding, in the Nordic trial (n = 518), there were 0 cases of endometrial cancer with an endometrial thickness of <= 4 mm: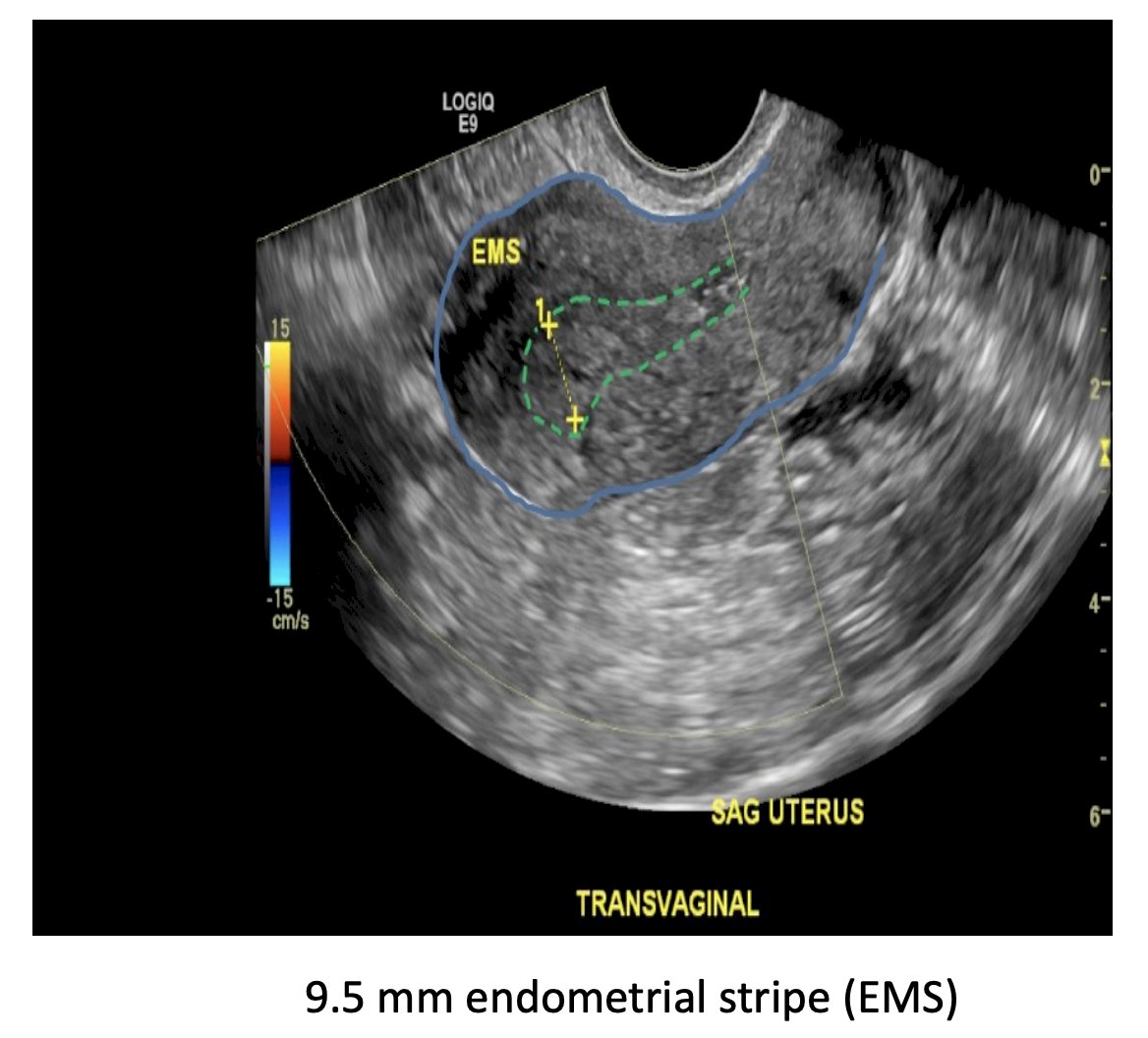
For pre-menopausal women that have vaginal bleeding, abnormal bleed is (i) if there is heavy bleeding (> 80 mL), (ii) bleeding between menstrual cycles, or (iii) bleeding for more than 21 days. Screening for endometrial cancer in asymptomatic women has been proven to not be cost-effective with a risk of ~0.7% - 3.2%.
With regards to uterine sarcoma, the incidence is <2 cases per 1,000 women, with the incidence decreasing: from 0.22% from 1983 – 2010 to 0.09% from 2000 – 2014. One risk factor for sarcoma, leading to an FDA warning in 2014 is power morcellation during a laparoscopic hysterectomy: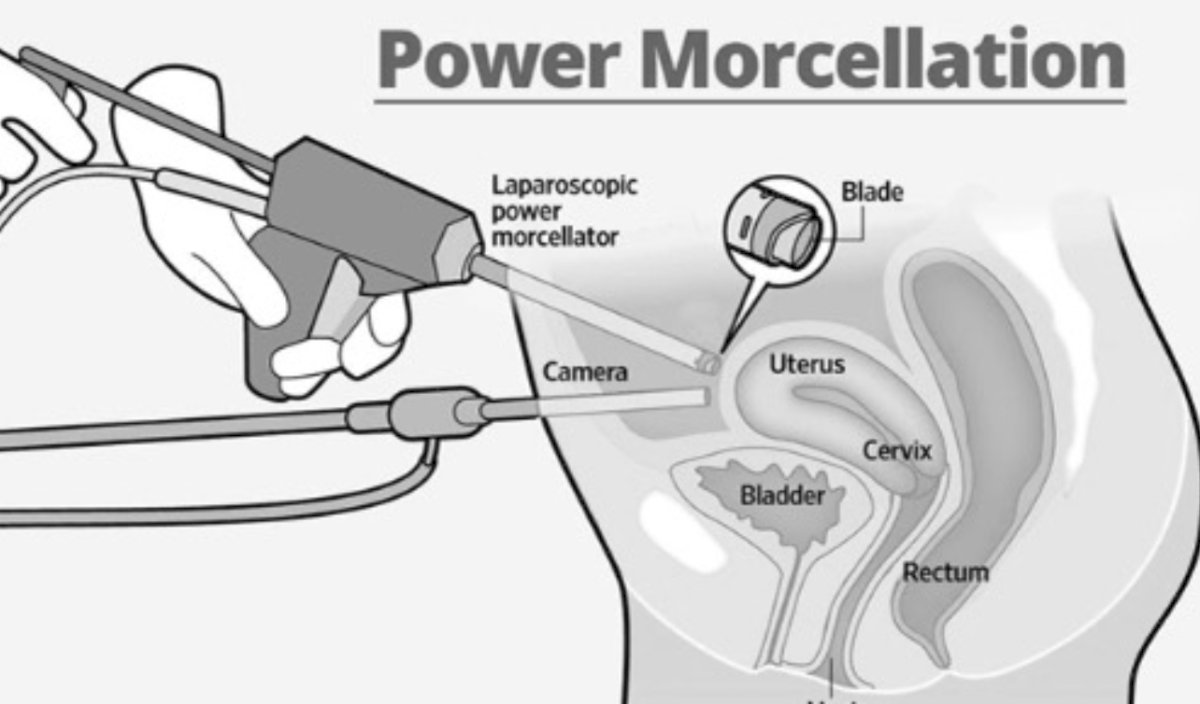
The warning was that 1 in 458 (0.22%) cases led to sarcoma, however, among the experts, the feeling is that this is more in line with 0.02-0.09% of cases.
Finally, Dr. Smith highlighted how we should be managing the adnexa. The benefit of oophorectomy is that it (i) decreases the risk of ovarian cancer to a lifetime risk of only 1.4%, (ii) leads to a 96% decrease in risk of mortality from ovarian cancer, and (iii) decreases the possibility of requiring adnexal surgery. However, the risks of oophorectomy include a 13% increased risk of mortality, which is highest in women < 50 years of age and lowest in women > 65 years of age. Thus, there is an important risk benefit consideration when deciding between a bilateral salpingo-oophorectomy at the time of urologic surgery: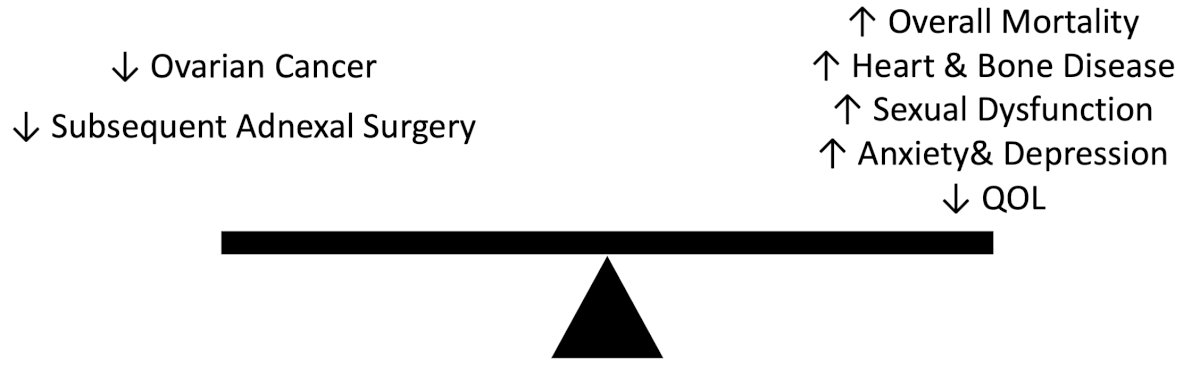
Dr. Smith notes that certain patients should have a bilateral salpingo-oophorectomy at the time of urologic surgery, include those with hereditary syndromes such as BRCA, Lynch syndrome, or a strong family history. Considerations for a salpingectomy are such that this is thought to be the origin of ovarian cancer, with precursor lesions in the tubes, thus a salpingectomy decreases the risk of ovarian cancer. Large studies suggested that a salpingectomy does not increase complications, leads to a 35% decreased risk of ovarian cancer, and does not decrease ovarian function. The risk benefit analysis for consideration of salpingectomy at the time of urologic surgery is as follows:
In 2018, Sussman et al. performed a survey of the Society of Urologic Oncology (SUO) members to assess the knowledge base and practice patterns of urologic oncologists with regard to management of the gynecological organs at the time of radical cystectomy.2 Response rate was 24% (159/660) among SUO members, of whom 110 (69%) were academic urologists and 58 (36%) involved in training urologic oncology fellows. Among participants, 75% had performed an ovarian-sparing radical cystectomy and only 14% were aware that salpingectomy alone reduces the risk of ovarian cancer. 95% were aware that bilateral salpingo-oophorectomy increases the risk of osteoporosis, 66% were aware that it increases the risk of cardiovascular disease, and 26% were aware that it increases the risk of all-cause mortality. Reasons for bilateral salpingo-oophorectomy at the time of radical cystectomy included concern for urothelial carcinoma metastasis (54%), development of future gynecologic pathology (50%), and facilitation of pelvic lymph node dissection (36%).
To summarize adnexal management, Dr. Smith re-emphasized:
- Oophorectomy: decreases risk of ovarian cancer, but increases risk of mortality
- Salpingectomy: decreases risk of ovarian cancer
- Urologists should counsel patients considering the risks and benefits
Dr. Smith concluded her presentation discussing gynecologic considerations for the urologic oncologist and optimizing care for women by again highlighting the key factors associated with optimized care:
- Gynecologic history
- Loss of femininity
- Risk of cancer
- Hormonal effects
- Sexual dysfunction

Presented by: Ariana L. Smith, MD, Penn Medicine, Philadelphia, PA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 Society of Urologic Oncology (SUO) Annual Meeting, Washington, D.C., Tues, Nov 28 – Fri, Dec 1, 2023.
References:
- Taylor BL, Matrai CE, Smith AL, et al. Gynecologic organ involvement during radical cystectomy for bladder cancer: Is it time to routinely spare the ovaries? Clin Genitourin Cancer. 2019 Feb;17(1):e209-e215.
- Sussman RD, Han CJ, Marchalik D, et al. To oophorectomy or not to oophorectomy: Practice patterns among urologists treating bladder cancer. Urol Oncol. 2018 Mar;36(3):90.e1-90.e7.


