(UroToday.com) The 2023 Society of Urologic Oncology (SUO) annual meeting held in Washington, D.C. between November 28th and December 1st, 2023, was host to a prostate cancer course. Dr. Philip Saylor discussed the use of bone supportive agents throughout a patient’s prostate cancer ‘journey’. The objectives of his presentation were to highlight:
- The differences between osteoporosis and bone ‘weakening’ secondary to bone metastases from prostate cancer
- Discuss osteoclast-targeted agents for bone metastases, including both zoledronic acid and denosumab
- Discuss special clinical treatment scenarios:
- Radiopharmaceuticals
- Ablative radiation (e.g., Stereotactic body radiotherapy)
- Synthesis and unanswered questions
Dr. Saylor began by noting that a skeletal-related event, or an SRE, is an ‘artificial problem’ that was created as an endpoint to be evaluated in clinical trials of bone-targeted medicines. The SRE definition includes:
- Pathologic fractures
- Surgery or radiation to the bone
- Spinal cord compression
Originally, SREs were considered a primary endpoint of trials evaluating bone-targeted therapy. However, the incidence of SREs has been more recently reported as a secondary endpoint in all major trials of drug therapy in the advanced prostate cancer space.
Osteoporosis, which refers to bone disease that develops when bone mineral density and bone mass decreases or when the quality or structure of bone changes, can lead to a decrease in bone strength that can increase the risk of skeletal fractures. All patients receiving androgen deprivation therapy (ADT) are at increased risk of fragility fractures secondary to osteoporosis. The real key in the ongoing management of these patients is to continually risk assess them using the FRAX® Fracture Risk Assessment Tool. These patients have many options which include:
- Alendronate administered weekly
- Denosumab every six months
- Zoledronic acid once yearly
Conversely, SREs occur in prostate cancer patients with bone metastases and often castration resistance. These patients require ‘high intensity’ treatment with potent agents administered monthly. The osteoclast is a validated therapeutic target in the management of prostate cancer patients to reduce the risk of SREs. Osteoclast inhibition with zoledronic acid (a bisphosphonate) or with denosumab (a monoclonal antibody to RANK ligand) reduces the risk for skeletal events in men with castration-resistant prostate cancer metastatic to the bone. Osteoclast inhibition with any of several bisphosphonates improves bone mineral density, a surrogate for osteoporotic fracture risk.1
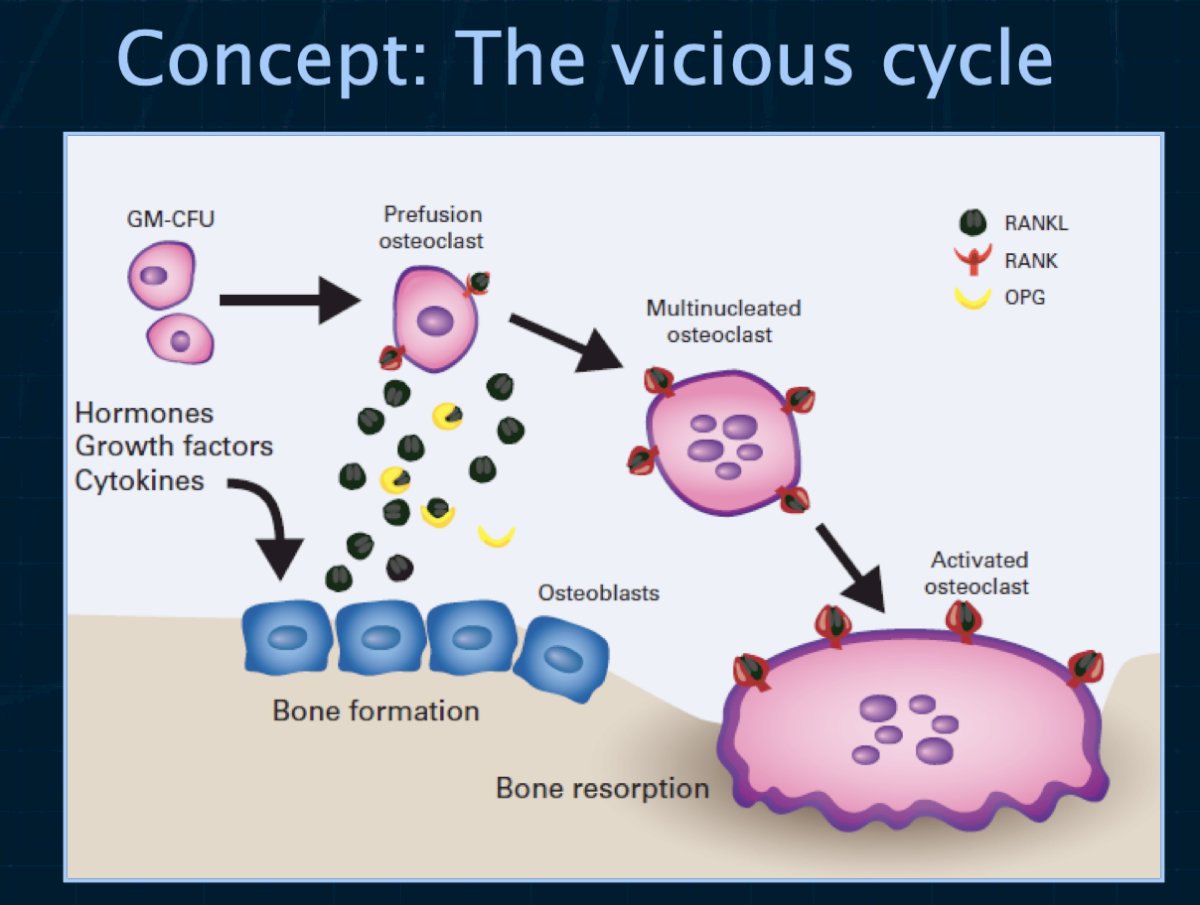
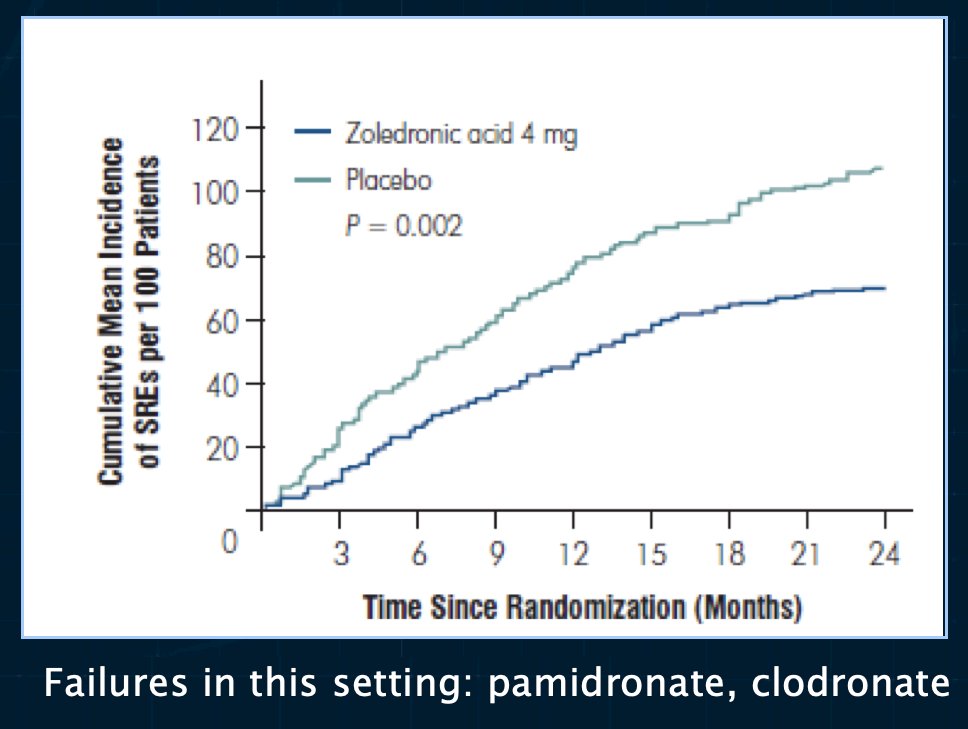
In 2002, Saad et al. demonstrated that the use of zoledronic acid, compared to placebo, in patients with hormone-refractory prostate cancer and a history of bone metastases decreased the incidence of SREs from 44% to 33%. Compared with urinary markers in patients who received placebo, urinary markers of bone resorption were statistically significantly decreased in patients who received zoledronic acid (p=0.001). Zoledronic acid at 4 mg given as a 15-minute infusion was well tolerated, but the 8 mg dose was associated with renal function deterioration. As such, Dr. Saylor emphasized that this drug should be infused over 15 minutes to minimize renal toxicity and a dose reduction should be considered for patients with stable mild renal insufficiency.
What about denosumab versus zoledronic acid? A phase 3 randomized controlled trial of 120 mg subcutaneous denosumab plus intravenous placebo versus 4 mg intravenous zoledronic acid plus subcutaneous placebo administered every 4 weeks in men with castration-resistant prostate cancer demonstrated that the median time to first on-study SRE was 20.7 months with denosumab compared with 17·1 months with zoledronic acid (HR=0.82, 95% CI: 0.71 to 0.95; p=0·0002 for non-inferiority; p=0·008 for superiority). There was no significant difference in overall survival outcomes.3 Dr. Saylor argued that these results suggest that both agents help, although denosumab may be better. Denosumab is significantly more expensive than zoledronic acid, and thus cost may be an issue in this situation.
When SREs were evaluated as a secondary endpoint, such as in the phase III AFFIRM trial of enzalutamide versus placebo in the post-docetaxel mCRPC setting, patients receiving enzalutamide had a significantly prolonged time to first SRE (16.7 versus 13.3 months).4 The key takeaway here is that an adequate response to systemic therapy in and of itself decreases the incidence of SREs.
While zoledronic acid and denosumab are well-established for the prevention of SREs in this advanced disease population, what are some agents that have not worked? Anything ‘weaker’ than these two agents, including pamidronate and clodronate, has been unsuccessful. Except for denosumab, which improves metastasis-free survival by 4.2 months, none of the bone protective agents have been demonstrated to prolong metastasis-free survival.
Important safety reminders when using these agents include:
- Osteonecrosis of the jaw, occurs with both zoledronic acid and denosumab. This risk is highest with invasive dental work while in therapy
- Hypocalcemia, which can be severe with either agent. Dr. Saylor noted that health providers should always check 25-OH Vitamin D levels before starting treatment and should supplement patients with calcium and Vitamin D during therapy with either agent.
In summary, Dr. Saylor noted that for patients with mCRPC and bone metastases, monthly therapy with either denosumab or zoledronic acid are acceptable treatment options. This is most essential when hormonal therapies are not working. Denosumab may be slightly more effective than zoledronic acid (time to SRE: 20.7 versus 17.1 months). We should always be aware of the risk of osteonecrosis of the jaw with time.
What about mCRPC patients receiving 223Radium? 223Radium is an alkaline earth metal that emits alpha particles and is administered intravenously monthly for six doses. In the ALSYMPCA trial, 223Radium was shown to prolong time to SREs, as well as OS.5 It remains unknown whether 223Radium can be safely paired with other active agents and what it does to bone health apart from cancer progression.
For patients receiving SBRT, ablative doses can be highly effective, particularly in the oligometastatic setting. Such patients are at a particularly high fracture risk after such therapy, making this a ‘rational’ setting antiresorptive agent use.
Presented by: Philip J. Saylor, MD, Assistant Professor, Department of Medicine, Massachusetts General Hospital, Boston, MA
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Society of Urologic Oncology (SUO) annual meeting held in Washington, D.C. between November 28th and December 1st, 2023
References:
- Saylor PJ, Lee RJ, Smith MR. Emerging therapies to prevent skeletal morbidity in men with prostate cancer. J Clin Oncol. 2011;29(27):3705-3714.
- Saad F, Gleason DM, Murray R, et al. A Randomized, Placebo-Controlled Trial of Zoledronic Acid in Patients With Hormone-Refractory Metastatic Prostate Carcinoma. J Nat Cancer Instit. 2002;94(19):1458-1468.
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768)”813-822.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. New Engl J Med. 2012;367(13):1187-1197.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223.


