(UroToday.com) The 2023 Society of Urologic Oncology (SUO) annual meeting held in Washington, D.C. between November 28th and December 1st, 2023, was host to a prostate cancer course. Dr. Evan Yu provided an overview of ongoing clinical trials in the metastatic castration-sensitive prostate cancer (mCSPC) disease space.
Dr. Yu began by highlighting the recent advances in the mCSPC disease space. While the CHAARTED1 and STAMPEDE2 trials established the role of doublet therapy with docetaxel + hormone therapy, this double combination is no longer recommended for the 1st line systemic treatment of such patients by the National Comprehensive Cancer Network (NCCN). This is due to the emergence of triplet therapy options and the more favorable side effect profile of androgen deprivation therapy (ADT) plus androgen receptor pathway inhibitor (ARPIs) combinations for mCSPC patients.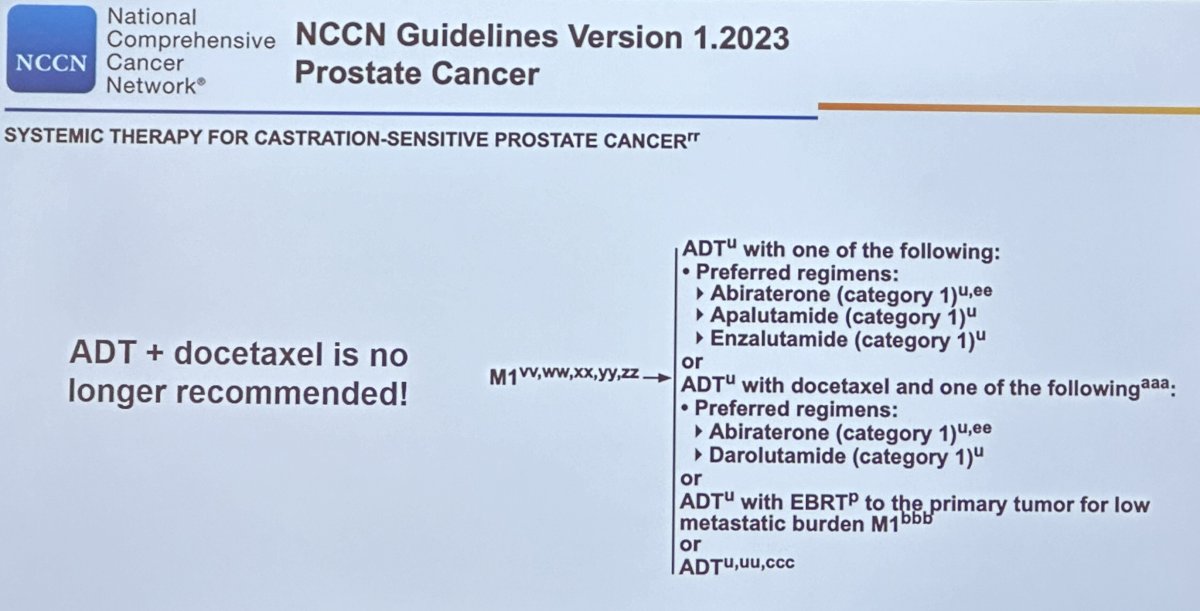
While continued investigation and ongoing clinical trials are crucial in this disease space, it is clear that the utilization of systemic therapy intensification, albeit doublet or triplet combinations, is clearly lagging in clinical practice. In a population-based study by Heath et al., real-world treatment patterns for 66,844 patients with mCSPC treated between January 2015 and January 2021 demonstrated high rates of utilization of ADT alone, especially among older adults. Only 25% of patients received an ARPI and 12% received chemotherapy.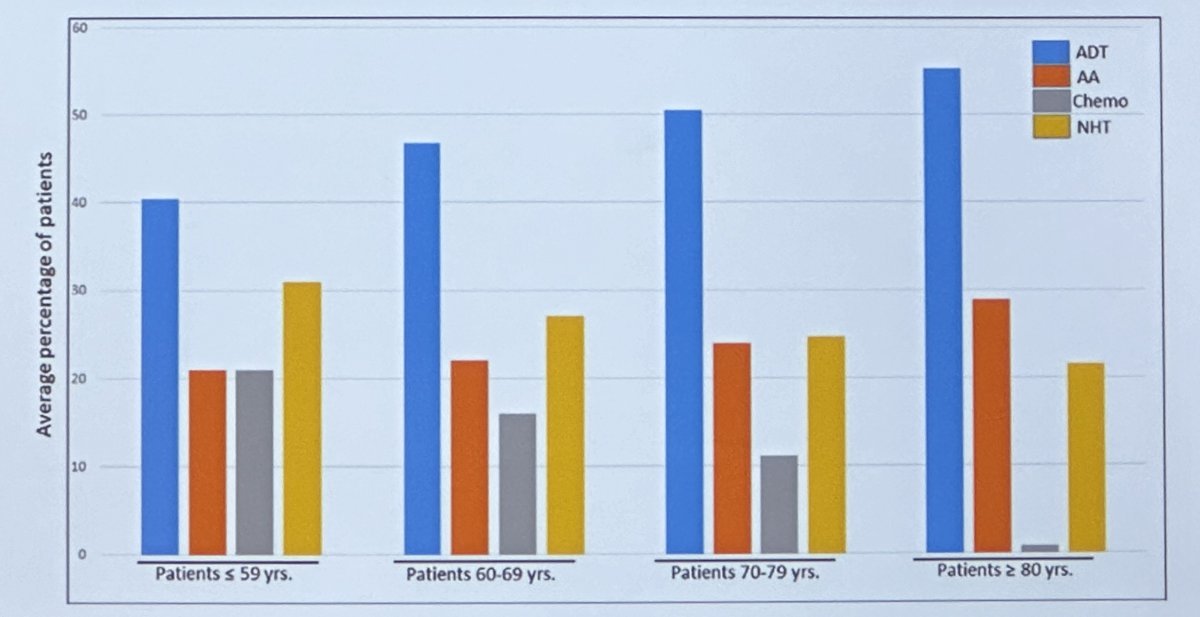
There are numerous unanswered questions that remain to be answered in the mCSPC disease space:
- What is the role/sequencing of immunotherapy?
- What is the role/sequencing of DNA repair deficiency agents (e.g., PARP inhibitors)?
- What is the role/sequencing of radioligand therapy?
- Are there other proliferation/survival pathways?
- How and for whom do we de-intensify treatment to improve patient quality of life?
- Do we need docetaxel as part of doublet or triplet therapy regimens?
Dr. Yu highlighted the following ongoing clinical trials of immunotherapy for mCSPC:
- NCT04633252: Phase 1/2 trial of M9241 with docetaxel
- NCT05733351: Phase 1 trial of vudalimab (XmAb20717) with abiraterone, enzalutamide, or abiraterone + docetaxel
- NCT04126070: Phase 2 of nivolumab + docetaxel for DNA repair deficient, immunoprofile inflamed, or biomarker negative cohorts
- NCT05189457: First strike, second strike, and consolidation with docetaxel plus tislelizumab (anti-PD1 antibody)
Notable ongoing clinical trials for mCSPC using DNA repair deficiency agents include:
- NCT04821622: Phase 3 trial of enzalutamide plus talazoparib versus placebo plus enzalutamide in patients with DDR gene mutated mCSPC (TALAPRO-3)
- NCT04497844: Phase 3 trial of niraparib in combination with abiraterone acetate plus prednisone (AAP) versus AAP for the treatment of patients with deleterious germline or somatic homologous recombination repair (HRR) gene-altered mCSPC.
- NCT04332744: Randomized (2:1) phase 2 trial of enzalutamide plus talazoparib vs. enzalutamide (ZZ-First)
- NCT04734730: Phase 2 of talazoparib with abiraterone
- NCT03934840: CASCARA Phase 2 trial of cabazitaxel + carboplatin followed by abiraterone
The PSMAddition study (NCT04720157) is a randomized phase 3 trial of 177Lu-PSMA-617 plus standard of care versus standard of care alone.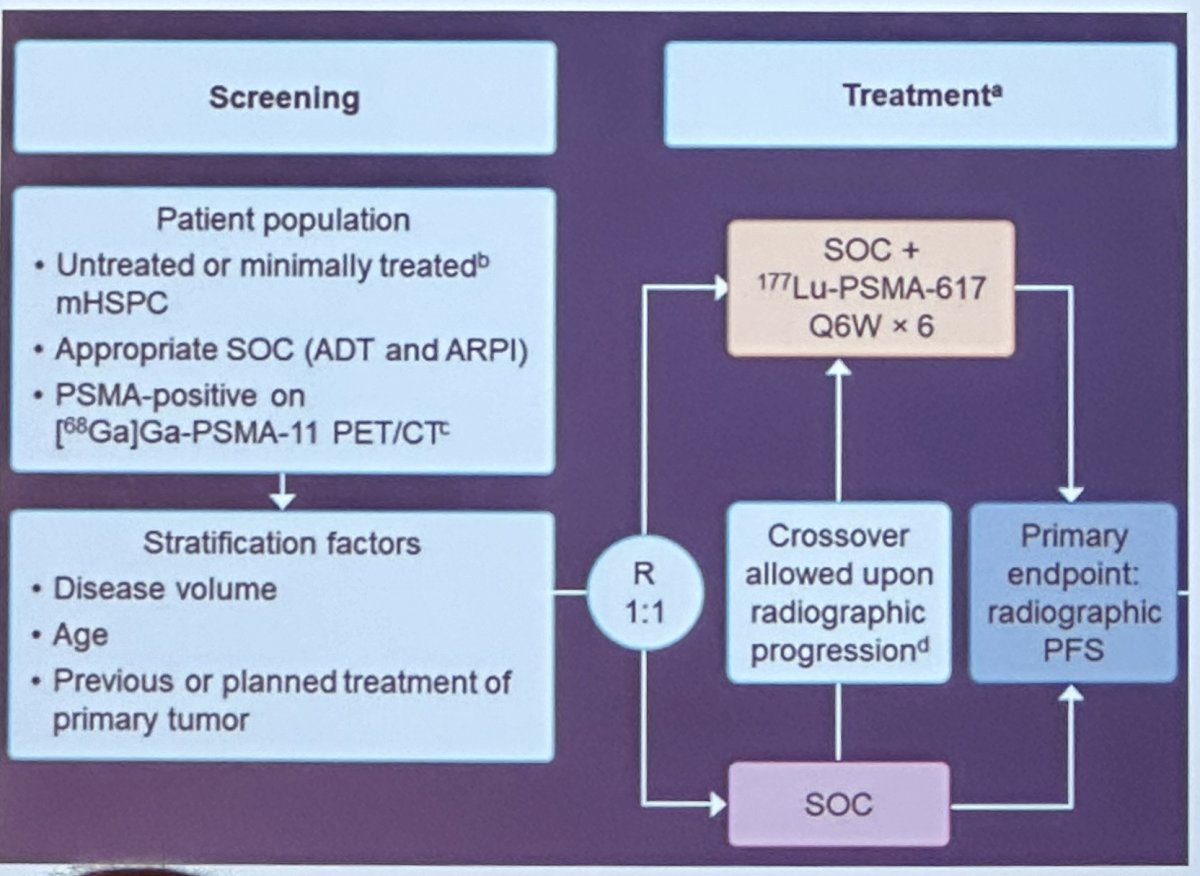
Ongoing clinical trials for mCSPC with proliferation/survival pathways include:
- NCT04493853: CAPItello-281 - Randomized phase 3 trial of capivasertib (PTEN inhibitor) + abiraterone vs. abiraterone for PTEN deficiency
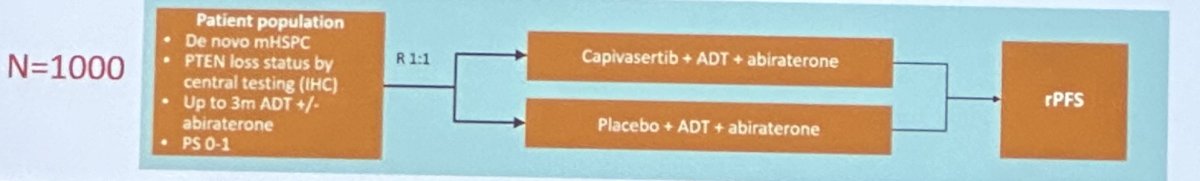
- NCT05288166: CYCLONE 3 - Randomized phase 3 trial of Abemaciclib + abiraterone vs. abiraterone for high risk mCSPC (visceral met(s) or 4 or more bone metastases by bone scan)
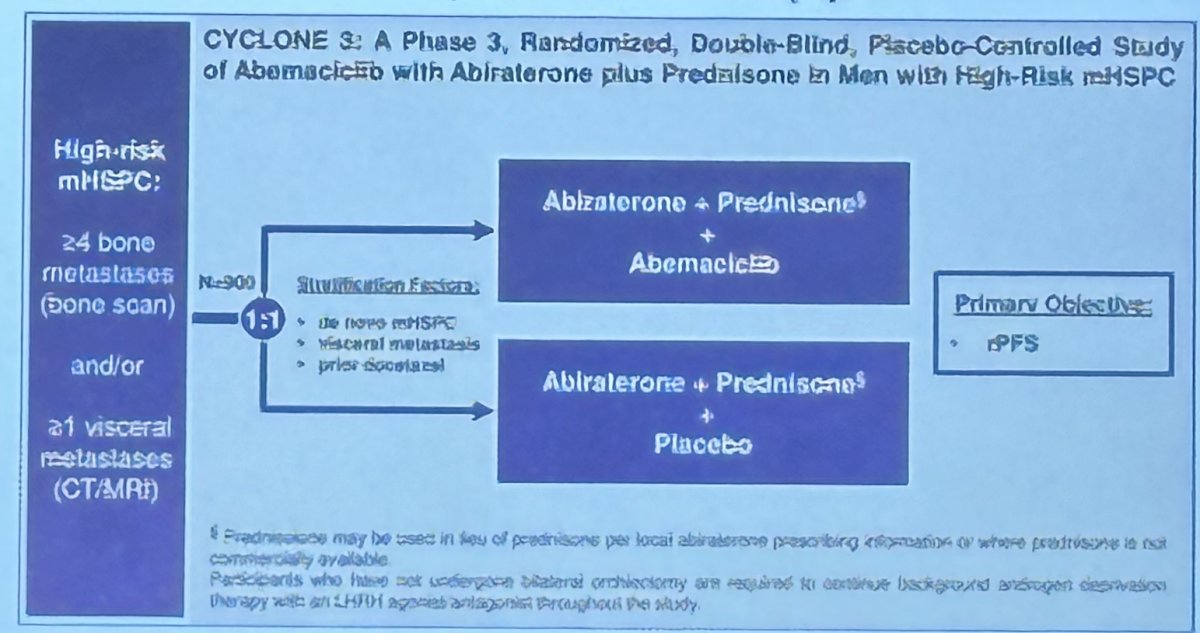
Notable ongoing trials that are evaluating treatment de-intensification strategies include:
- NCT05884398: LIBERTAS - randomized Phase 3 trial of Apalutamide with intermittent ADT versus Apalutamide with continuous ADT
- NCT05241860: A-DREAM - phase 2 trial of interrupting hormonal therapy for exceptional PSA responders
- NCT04666129: Phase 1 trial of relugolix in mCSPC or mCRPC in combination with abiraterone, apalutamide, or docetaxel
With regards to the need for docetaxel as part of systemic therapy intensification, NCT06060587 is a randomized phase 2 trial that compares abiraterone to abiraterone + docetaxel.
Dr. Yu concluded with the following take home messages:
- We have very good treatment options for patients with mCSPC, but we need to encourage more treatment intensification in clinical practice
- Triplet therapy is appropriate for patients with adverse features e.g., de novo high volume metastatic disease
- Opportunities exist for niche and precision medicine trials e.g., immunotherapy, radioligand therapy, and biomarker selection trials
- Treatment de-intensification / quality of life trials are ongoing, and more are needed
- Do we need docetaxel as part of early treatment intensification? This remains to be answered.
Presented by: Evan Yu, MD, Professor of Medicine, Division of Oncology, Department of Medicine, Seattle, WA
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Society of Urologic Oncology (SUO) annual meeting held in Washington, D.C. between November 28th and December 1st, 2023
References:- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373(8):737-746.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177.
- Heath EI, Dyson GE, Cackowski FC, et al. Treatment Intensification Patterns and Utilization in Patients with Metastatic Castration-Sensitive Prostate Cancer. Clin Genitourin Cancer. 2022;20(6):524-532.


