(UroToday.com) The 2023 Society of Urologic Oncology (SUO) annual meeting held in Washington, D.C. between November 28th and December 1st, 2023, was host to a prostate cancer course. Dr. Scott Tagawa discussed the current state and future applications of targeted radionuclides for the treatment of metastatic castrate-resistant prostate cancer (mCRPC).
Dr. Tagawa began by noting that prostate-specific membrane antigen (PSMA)-targeted radionuclide therapy involves the intravenous administration of systemic radioisotopes which are taken up by PSMA-expressing cells. The radionuclide component is a radioactive particle which is typically either a β (e.g., 177Lu-PSMA-617)- or an α-emitter (e.g., 223Radium). The efficacy and toxicity of these agents is partially related to their inherent structural properties. The PSMA-targeting vehicle is the ‘conduit’ for these agents, with different properties mostly related to their size. This affects their kinetics and biodistribution.
Examples of PSMA-targeted radionuclide therapeutics that have been studied in clinical trials include:
- 177Lu-PSMA-617 (177Lu vipivotide tetraxetan)
- 177Lu-PSMA I&T (aka PNT2002)
- 177Lu-J591 (177Lu-rosopatamab aka TLX591)
- 131MIP-1095
- 177Lu-PSMA-R2
- 225Ac-J591
- BAY 2315497 (PSMA-TTC / 227Th-PSMA)
- 225Ac-PSMA-617
- 225Ac-PSMA I&T
- 177Lu-rhPSMA-10.1
- 67Cu SAR-PSMA
- 161Tb-PSMA-I&T
- 225Ac-“Trilium” PSMA
Dr. Tagawa next posed the question of whether β-emitting PSMA-targeted radioligand therapy is ready for ‘prime time’? There are two prominent trials in this space: TheraP and VISION.1,2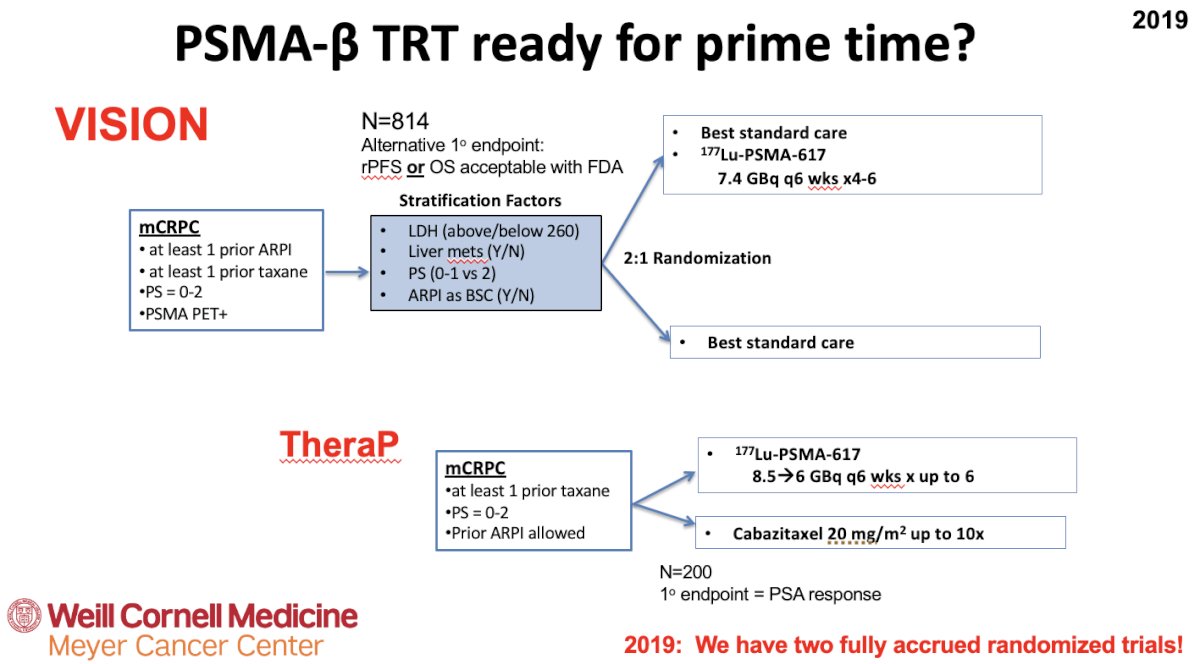
TheraP was the first randomized study to evaluate 177Lu-PSMA-617 versus cabazitaxel for men with mCRPC after docetaxel. In this open label, phase II trial, 200 men were randomized to either 177Lu-PSMA-617 or cabazitaxel. To screen into the study, all men had both 68Ga-PSMA-11 and 18F-FDG PET/CT and were required to have high PSMA-expression (at least one site with SUVmax ≥20) and no sites of FDG-positive/PSMA-negative disease. All patients had progressive disease with rising PSA ≥20 ng/mL after docetaxel and 91% had received prior enzalutamide or abiraterone. Overall, 200 patients were randomized 1:1 to 177Lu-PSMA-617 at a dose of 6-8 GBq every 6 weeks for up to 6 cycles of therapy or cabazitaxel 20 mg/m2 every 3 weeks for up to 10 cycles. Patients were stratified based on disease burden and prior anti-androgen therapy. Of note, about 1/3 of patients who had registered for the study (91/291) were ineligible prior to randomization, either because of low PSMA expression or FDG discordant disease. The primary endpoint of this study was a PSA decline of 50% (PSA50) and secondary endpoints included PSA progression-free survival (PSA-PFS) and overall survival. After a median follow up of 13 months, 177Lu-PSMA-617 significantly improved PSA-PFS compared with cabazitaxel (HR: 0.63, 95% CI: 0.46 to 0.86) and a had a much higher PSA50 rate (66% versus 37%):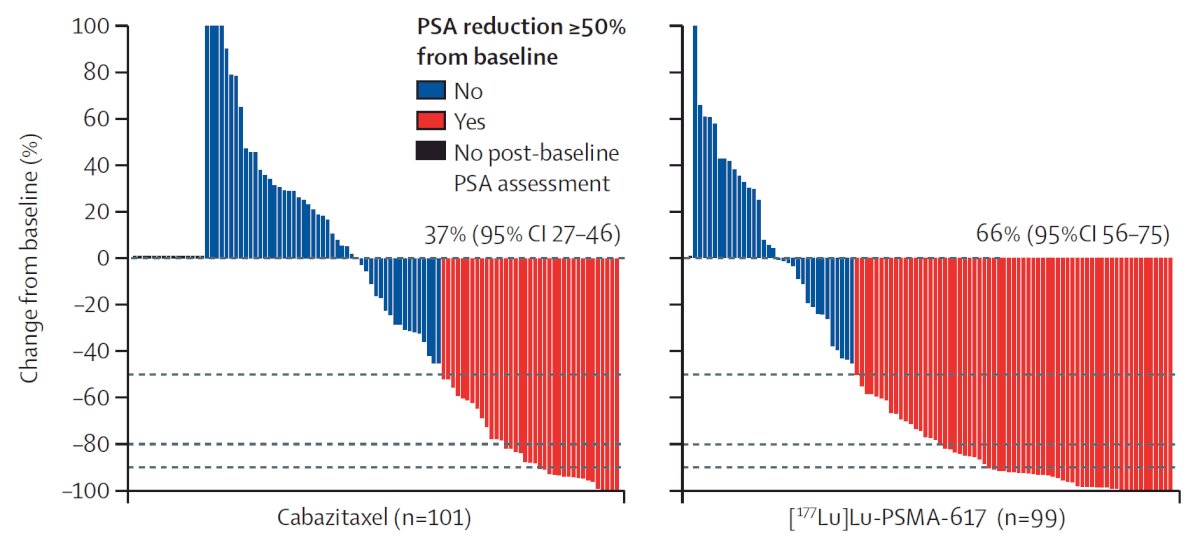
Additionally, there were superior RECIST response rates (33% versus 53%). In terms of the AE/safety profile, Grade 3/4 toxicity was seen in 54% of men on cabazitaxel compared to 35% of patients who received 177Lu-PSMA-617. Rates of thrombocytopenia, dry mouth, and dry eyes were seen more frequently in patients receiving 177Lu-PSMA-617, as expected due to normal PSMA expression in the salivary and lacrimal glands.
Updated analysis was presented at ASCO 2022 after a median follow-up of 36 months and PFS continued to favor the 177Lu-PSMA-617 arm (HR: 0.62, 95% CI: 0.45 to 0.85). There were no significant differences in restricted mean survival time overall survival between the two arms (19.1 months in 177Lu-PSMA-617 arm versus 19.6 months in cabazitaxel arm, 95% CI for difference: -3.7 to +2.7).
Following on the heels of TheraP, VISION was an international, randomized, open-label phase III study that evaluated 177Lu-PSMA-617 in men with PSMA-positive mCRPC who had previously received treatment with a next-generation androgen receptor signaling inhibition (abiraterone, enzalutamide, etc.) and one or two prior lines of taxane chemotherapy (NCT03511664). Importantly, patients must have had PSMA-positive disease on the basis of a central review of 68Ga-PSMA-11 staging scans. PSMA positivity was defined as uptake greater in metastatic lesions than in the liver. Further, they could have no PSMA-negative metastatic lesions. Following enrollment, patients were randomized in a 2:1 fashion to receive either 177Lu-PSMA-617 plus standard of care or standard of care alone.
The authors assessed two alternate primary endpoints: (i) radiographic progression-free survival (rPFS) using the Prostate Cancer Working Group 3 (PCWG3) criteria by independent central review and (ii) overall survival. Over a median follow-up of 20.9 months, treatment with 177Lu-PSMA-617 + standard of care significantly improved overall survival by a median of 4 months (15.3 versus 11.3 months; HR 0.62, 95% CI: 0.52 to 0.74; p < 0.001), compared to standard of care alone, in the overall cohort of all randomized patients (n=831):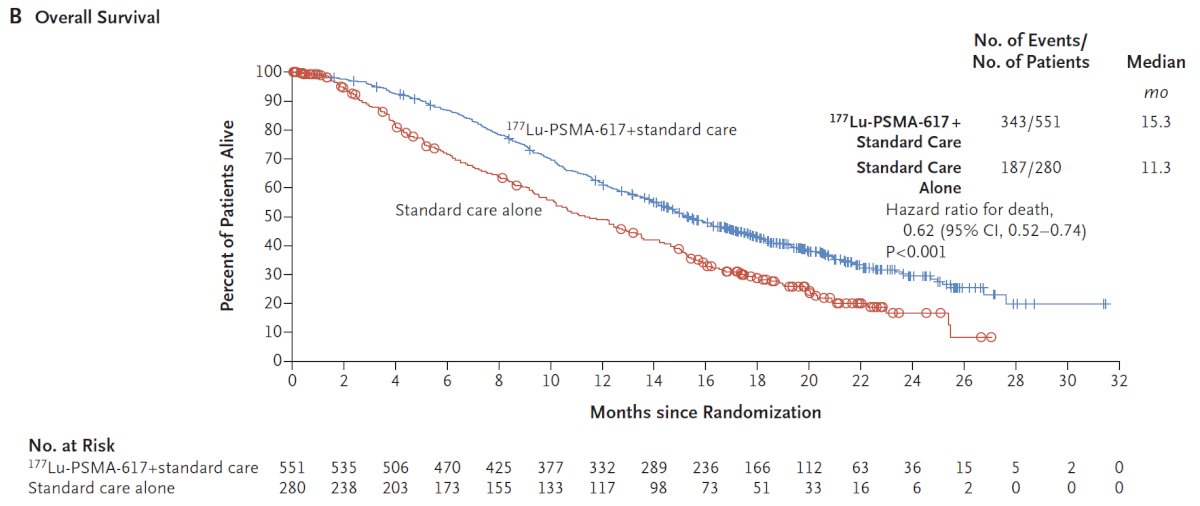
With regards to the other primary endpoint of rPFS, treatment with 177Lu-PSMA-617 + standard of care significantly improved rPFS by a median 5.3 months (8.7 versus 3.4 months; HR: 0.40, p<0.001):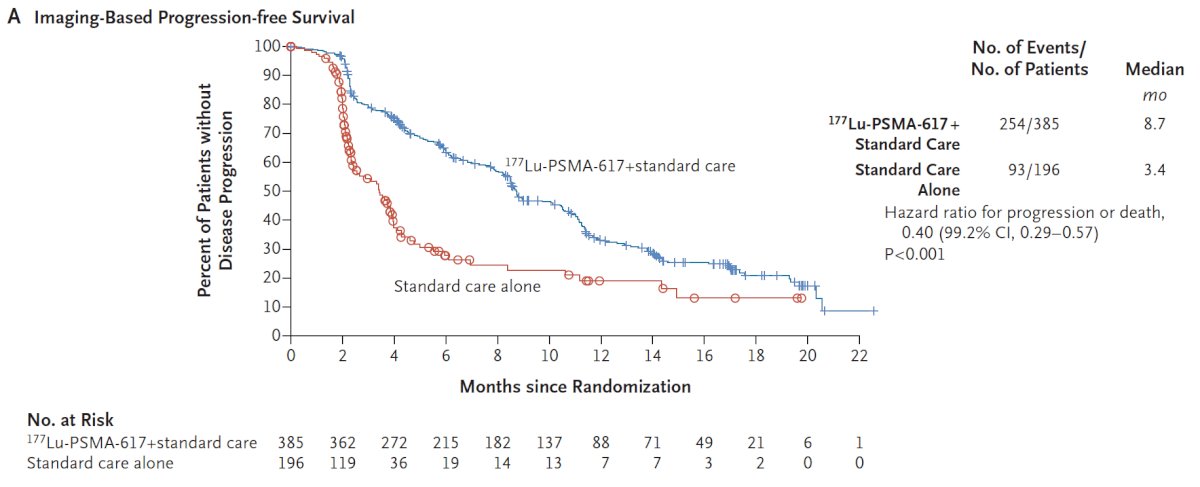
Can PSMA imaging findings be used as biomarkers for predicting treatment response? Dr. Tagawa noted that treatment pre-selection based on PSMA-PET/CT findings can likely improve the therapeutic ratio, particularly for small-molecule-carried beta radionuclides. However, he did note that patients with poor pre-treatment imaging predictors can still respond. Ad hoc analysis of the TheraP trial demonstrated that the magnitude of PSMA uptake is a predictive biomarker - in patients with PSMA SUVmean levels of 10 of greater, the odds of a response to 177Lu-PSMA-617 were substantially higher (OR: 12.2, 95% CI: 3.4 to 59.0) as compared to those with PSMA SUVmean of less than 10 (OR: 2.2, 95% CI: 1.1 to 4.5, p=0.03). This was reflected in superior PSA50 response rates (91% versus 52%) and PSA-PFS hazard ratios (0.45 versus 0.77) for SUVmean ≥ 10 versus SUVmean < 10. Metabolic tumor volume on FDG-PET was another predictive biomarker, with volume ≥ 200 mL portending significantly worse treatment response rates with both 177Lu-PSMA-617 and cabazitaxel.
While these results highlight the potential of PET/CT for pre-treatment selection, with other key advantages including assessment of intertumoral heterogeneity and evaluating biodistribution and kinetics of targeted agents for future drug development, notable drawbacks include:
- Limited to a ‘tumoral snapshot’ with tumor assessment at a moment in time only
- Dependent upon target size as well as target expression
- Some therapeutic constructs may be better for micrometastatic disease
PSMA can also be used to assess response (volume and expression), with this remaining an area of ongoing research.
Notably, with the trials of 177Lu-PSMA-617, there were no unexpected or concerning safety signals in the VISION or TheraP trials. 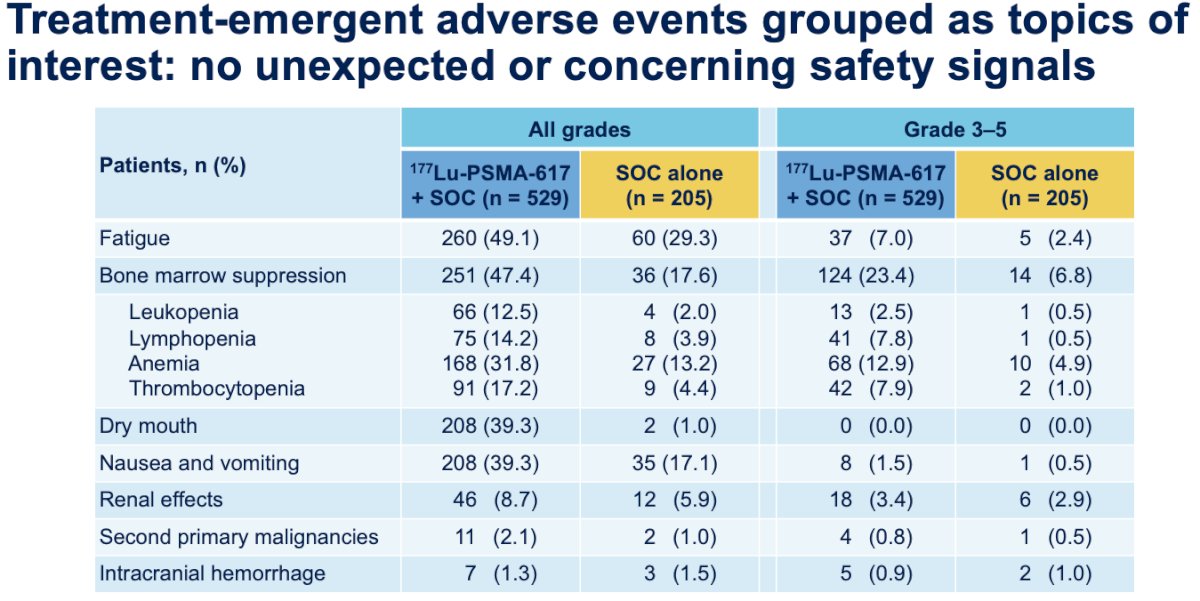
Ad hoc analysis of the VISION trial demonstrated that the addition of 177Lu-PSMA-617 to standard of care significantly improved health-related quality of life and pain scores, as assessed by the FACT-P and BPI-SF questionnaires.3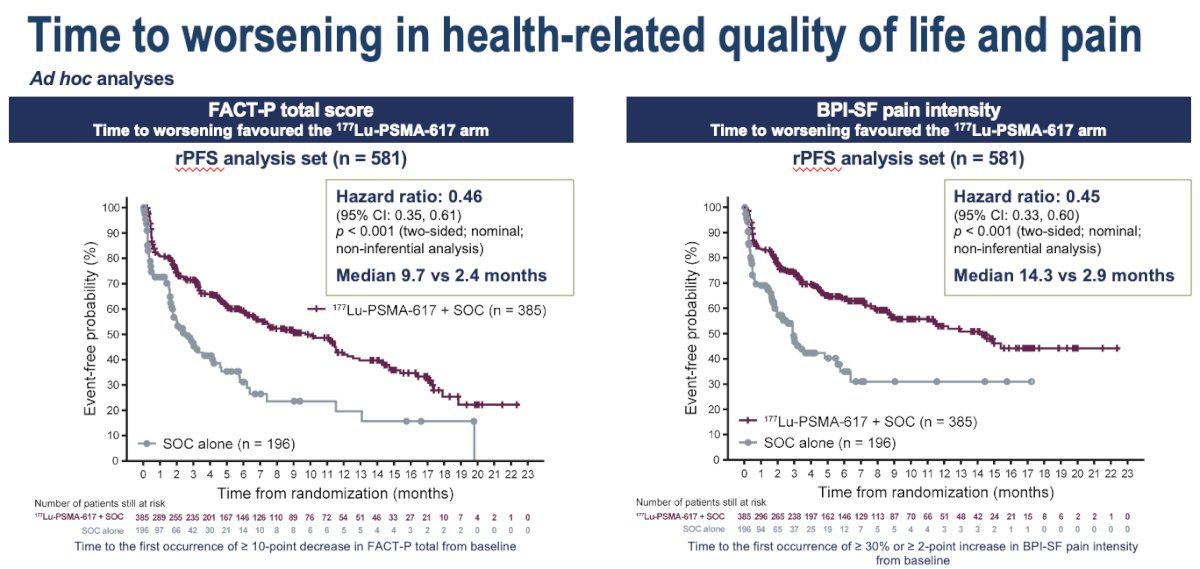
Furthermore, in the TheraP trial, the incidence of febrile neutropenia was significantly higher in the cabazitaxel arm (8%) and was non-existent with Lu-PSMA (0%). Of reported Lu-PSMA adverse events, the most common were grade 1 dry mouth (75%) and dry eyes (88%). There were no grade 5 events related to either drug.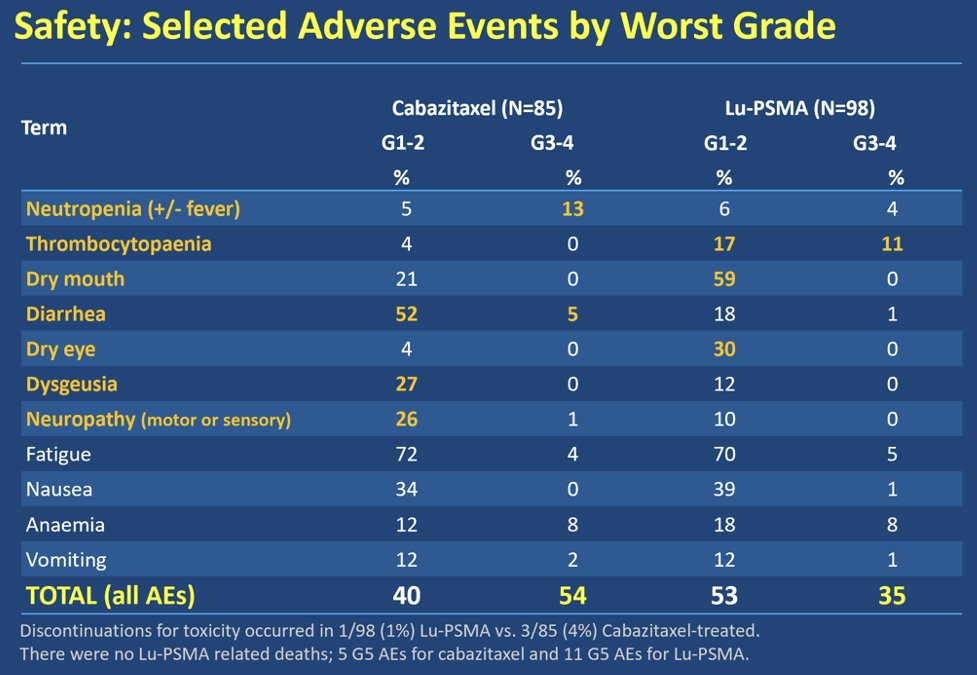
In an important recent advance, Dr. Gudenkauf and colleagues have developed a novel patient-reported outcomes measure for prostate cancer patients receiving radionuclide therapy,4 detailed below: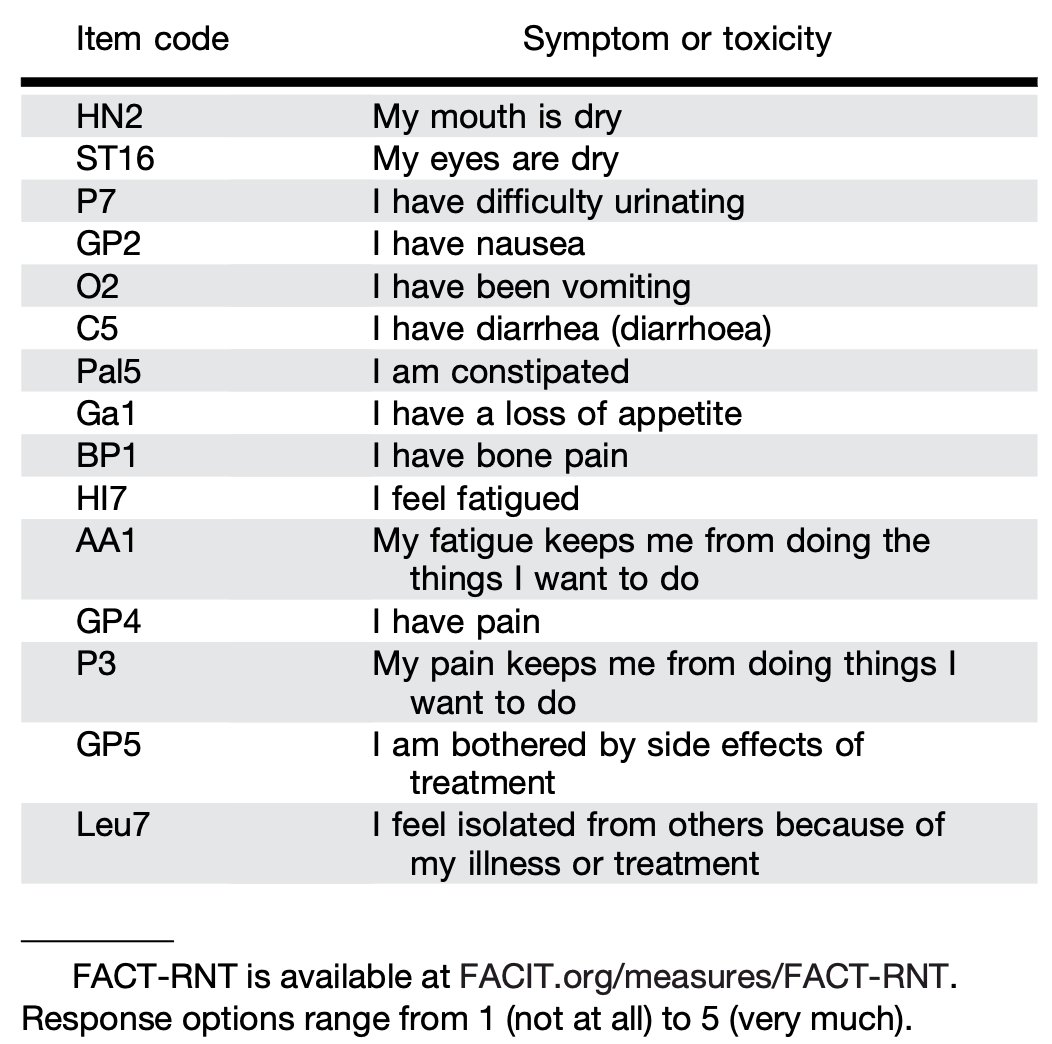
While it does appear that most adverse events are manageable and treatment with 177Lu-PSMA-617 is associated with quality-of-life benefits, long-term or delayed adverse events may be important, even those that are low-grade:
- Grade 1 platelets = 75 - 149K
- Some drugs and many clinical trials exclude patients with platelet counts < 100K, nearly all exclude Grade 2
Renal failure may also be a late event, and serum creatinine is not necessarily the best measure of renal function in isolation.
Remaining challenges with PSMA-targeted radionuclide therapy include:
- Optimizing the dose and schedule of small molecules
- Optimizing the patient population most likely to benefit from treatment:
- Pre-treated mCRPC: what are the ideal clinical, genomic, and radiographic factors for patient selection?
- Can we use these drugs in earlier mCRPC states (e.g., pre-docetaxel: SPLASH, PSMAfore)
- Can we use them in the non-castrate state? (e.g., PSMAddition/AFT53, UpFront PSMA)
- Can these agents be used in combination with other classes of drugs?
- Androgen receptor inhibitors, immunotherapy, chemotherapy, PARP inhibitors
Dr. Tagawa highlighted numerous ongoing trials that are evaluating PSMA—β targeted radioligand therapy in earlier mCRPC states:
- 177Lu-PSMA-617
- PSMAfore (NCT04689828)
- ENZA-p (NCT04419402; Randomized Phase 2)
- 177Lu-PSMA I&T
- SPLASH (NCT04647526), ECLIPSE (NCT05204927)
- 177Lu-TLX591
- ProstACT (NCT04876651)
In general, Dr. Tagawa noted that these are all phase 3 trials in the mCRPC setting following progression with one prior ARPI that are randomizing patients to 177Lu-PSMA small ligand molecule versus a 2nd ARPI or 177Lu-PSMA monoclonal antibody versus a 2nd ARPI or docetaxel, with rPFS as the primary endpoint.
The initial results of PSMAfore were recently presented at ESMO 2023. This trial included patients with mCRPC who were candidates for an ARPI change following progression with one prior ARPI and had ≥1 PSMA positive lesions and no exclusionary PSMA negative lesions by 68Ga-PSMA-11 PET/CT. Patients were randomized 1:1 to open-label 177Lu-PSMA-617 or an ARPI change (abiraterone or enzalutamide). The trial design for PSMAfore is as follows: 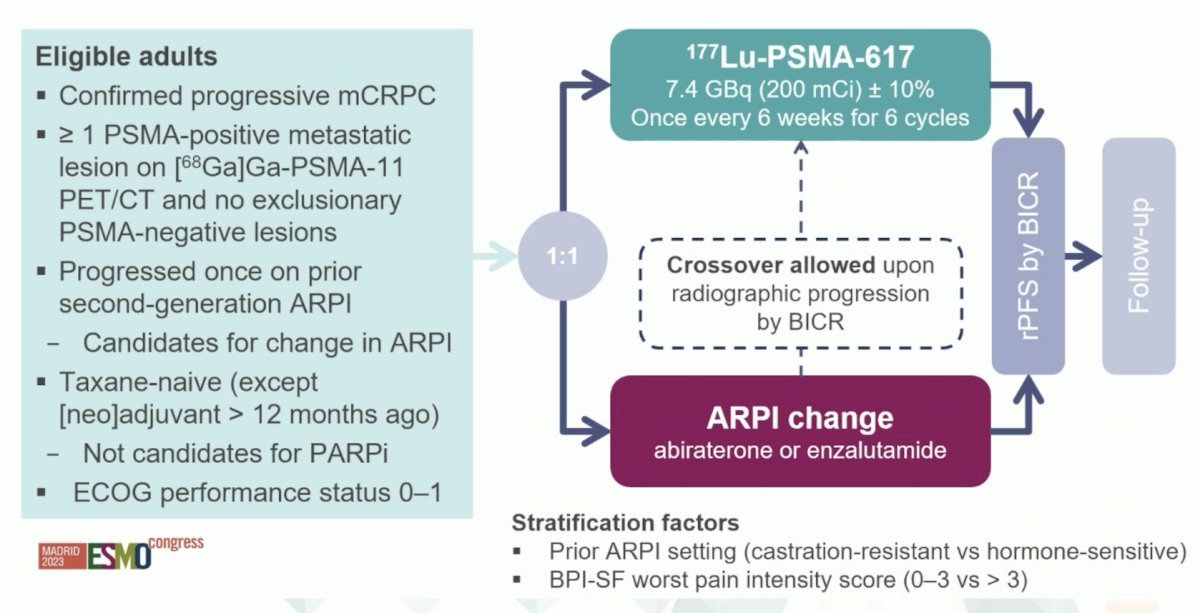
At the primary analysis (median follow-up, 7.3 months; n = 467), the primary endpoint of rPFS was met (HR: 0.43, 95% CI: 0.33 to 0.54):
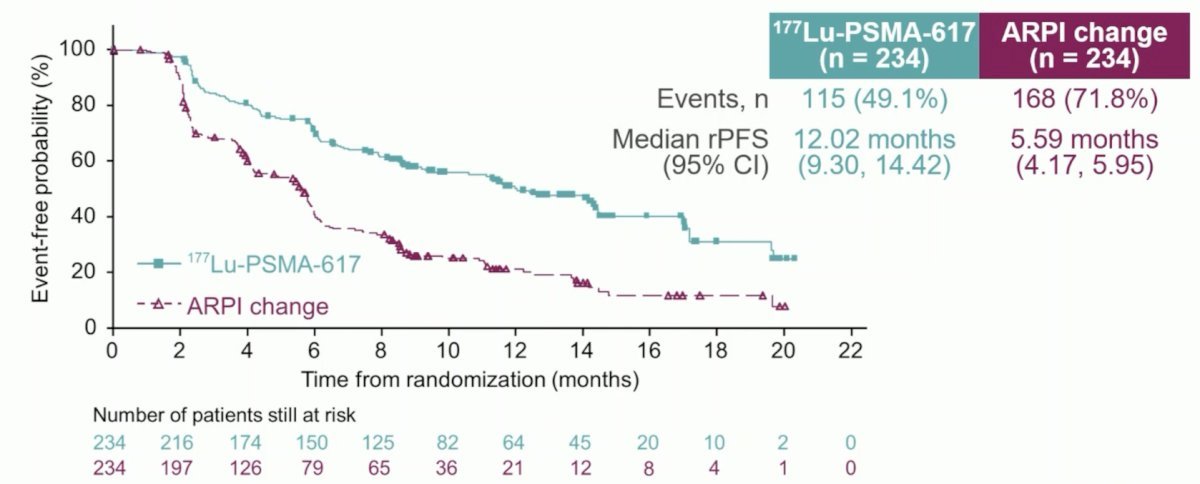
Additional efficacy endpoints similarly favored 177Lu-PSMA-617:
- Objective response rate: 51% vs 15%
- Confirmed PSA50: 58% vs 20%
- Time to symptomatic skeletal event: HR 0.35
- Time to FACT-P deterioration: HR 0.59
- Time to BPI-SF deterioration: HR 0.69
- Overall Survival (interim)
- No difference ITT population: HR 1.16 [95% CI 0.83 – 1.64]
- No difference after censoring for crossover (84.2% crossover):
- HR 0.80 (95% CI 0.48 – 1.33)
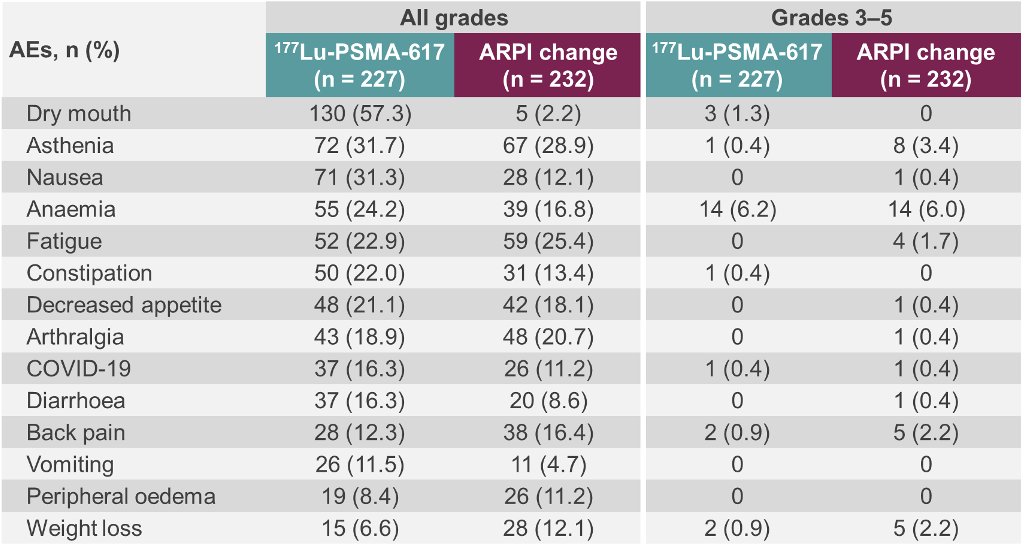
With regards to high-grade treatment-emergent adverse events, these were as follows (177Lu-PSMA-617 versus ARPI switch):
- Grade 3 – 4: 34% versus 43%
- Serious events: 20% versus 28%
- Fatal events: 1.8% versus 2.2%
- Adverse events leading to dose adjustment: 3.5% versus 15%
- Adverse events leading to treatment discontinuation: 5.7% versus 5.2%
Notable ongoing studies of PSMA-β targeted radioligand therapy in the mCSPC space are as follows: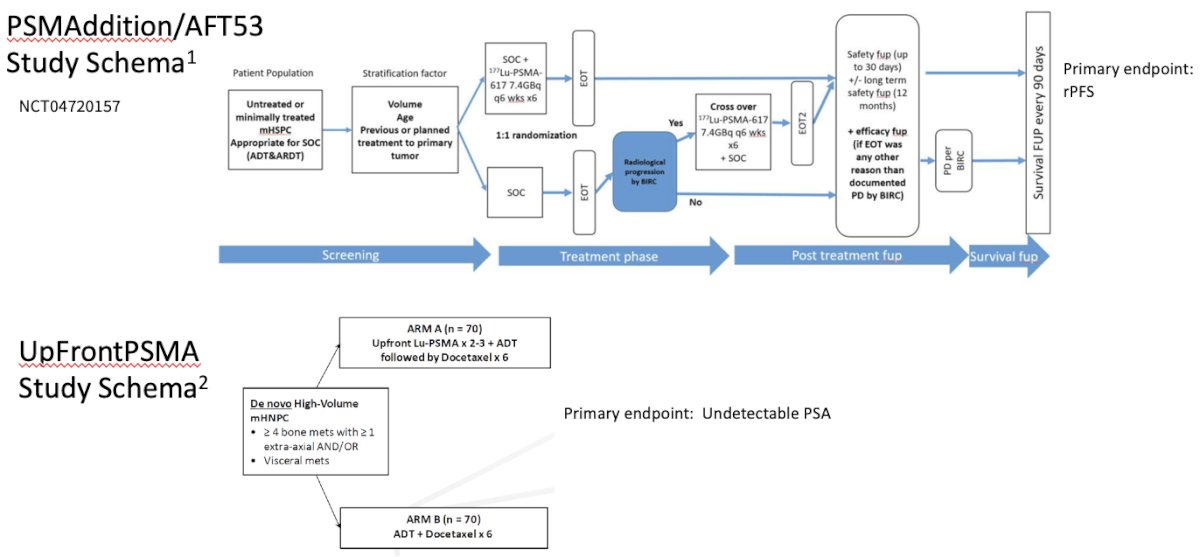
Notable combination trials of PSMA-β targeted radioligand therapy plus other agents, include:
- NCT00916123: dose-escalation 177Lu-J591 + docetaxel
- 177Lu-PSMA-617 + idronoxil
- NCT03805594 177Lu-PSMA-617 + pembrolizumab
- NCT03658447 PRINCE 177Lu-PSMA-617 + pembrolizumab
- NCT03874884 LuPARP 177Lu-PSMA-617 + Olaparib
- *NCT04419402 ENZA-p (enzalutamide +/- 177Lu-PSMA-617)
- NCT04886986 177Lu-PSMA I&T + 225Ac-J591
- NCT04946370 Pembrolizumab and ARSI +/- 225Ac-J591
- NCT05340374 LuCAB (177Lu-PSMA-617 + cabazitaxel)
- NCT00859781 ketoconazole + 177Lu/111In-J591 (M0 CRPC)
Beyond beta emitters, alpha emitters have shorter ranges of action and have significantly higher linear energy transfer. Dr. Tagawa noted that similar to β- small molecules, α-emitters have initially been evaluated in small retrospective series. There are now numerous ongoing academic prospective trials of these agents, with a recently presented phase 1 study demonstrating the safety and preliminary efficacy of such agents. Currently, multiple PSMA-targeted α-emitters are in development, including multiple antibodies and small molecules such as 225Actinium.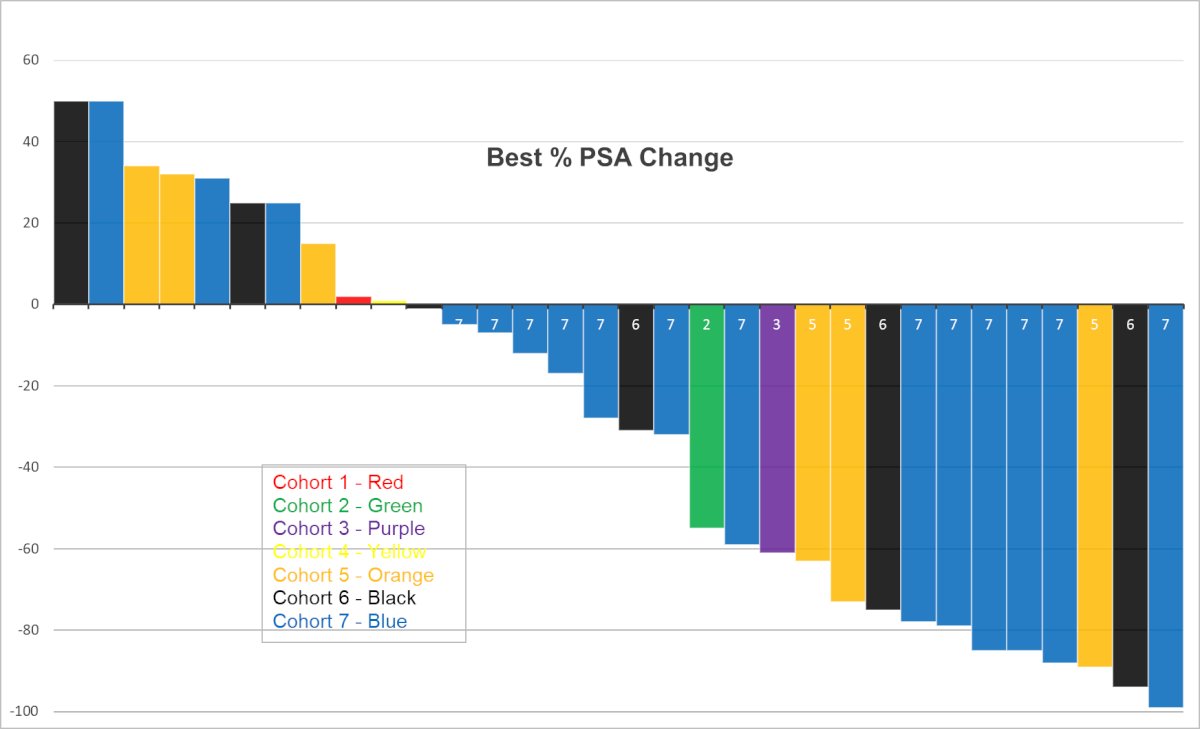
Dr. Tagawa concluded as follows:
- PSMA is a clinically-validated, consistent cell-surface target, that remains relevant in the current era
- Both small molecules and antibodies accurately target PSMA+ cells, can be radiolabeled, and have different kinetics and biodistribution
- Prostate cancer is radiosensitive; dose-response data exist
- We can exploit the selectivity of PSMA expression to deliver high doses of radioactive particles (or drugs) to tumors with relative sparing of normal organs
- Can we get a high enough dose to all tumors for cure? [heterogeneity noted]
- Many potential combinations (androgen receptor, taxane, immune checkpoint inhibitors, PARP, etc.)
- Can we pre-select the optimal patient population (imaging, genomics, etc)?
Presented by: Scott T. Tagawa, MD, MS, FACP, FASCO, Professor of Medicine, Department of Medicine, Weill Cornell Medicine, New York, NY
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Society of Urologic Oncology (SUO) annual meeting held in Washington, D.C. between November 28th and December 1st, 2023
References:
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385:1901-1103.
- Fizazi K, Herrmann K, Krause BJ, et al. Health-related quality of life and pain outcomes with [177Lu]Lu-PSMA-617 plus standard of care versus standard of care in patients with metastatic castration-resistant prostate cancer (VISION): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24(6):597-610.
- Gudenkauf LM, Chavez M, Maconi ML, et al. Developing a novel patient reported outcomes measure for prostate cancer patients receiving radionuclide therapy. J Nucl Med. 2023;64(6):869-872.


