(UroToday.com) At the 2023 Society of Urologic Oncology (SUO) annual meeting held in Washington, D.C. between November 28th and December 1st, 2023, Dr. Michael Cookson was honored with the prestigious Huggins Medal award.
Dr. Cookson began by highlighting the SUO’s achievements over the past four decades. Founded in 1984, notable achievements by this society have included:
- 2000: The 1st annual winter meeting at The National Institute of Health
- 2000: The SUO accredited fellowships were formalized
- 2008: The Clinical Trial Consortium was founded

But none of this would have been possible without the contributions of the person after whom this award is named: Dr. Charles Huggins. In 1941, Dr. Huggins, along with Dr. Clarence Hodges, reported that prostate cancer was an androgen-driven/dependent disease, with androgen receptors highly expressed on prostate cancer cells. This culminated in the use of androgen deprivation as the primary therapy for metastatic disease.
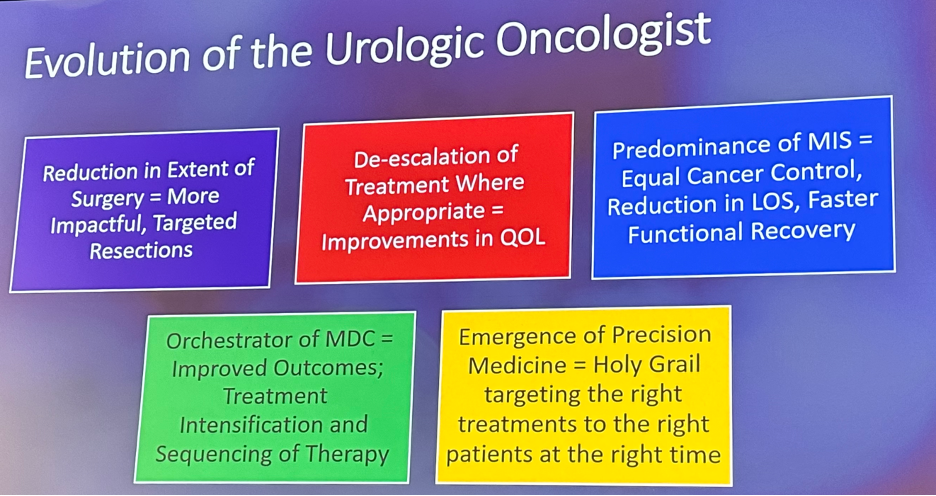
Since then, we have observed a number of significant advances in the field of urologic oncology and this has both directly and indirectly led to the evolution of the urologic oncologist’s role, summarized by these five main domains:
- Reduction in the extent of surgical resections
- De-escalation of treatment where appropriate
- Predominance of minimally invasive surgery
- Orchestrator of multidisciplinary care model
- Emergence of precision medicine
Historically, there have been numerous theories proposed to describe cancer invasiveness and disease progression. The “Halstead Theory” of cancer metastasis was proposed by Dr. William Halstead. He proposed that "although breast cancer begins as a local disease, it spreads in a contiguous manner away from the primary site through the lymphatic system". This led to an emphasis on aggressive locoregional treatment to prevent further spread. In a quote that emphasizes the contrasting priorities, current advances, and shifting goals in contemporary oncologic care, Dr. Halstead noted: "Disability, ever so great, is a matter of very little importance as compared with the life of the patient."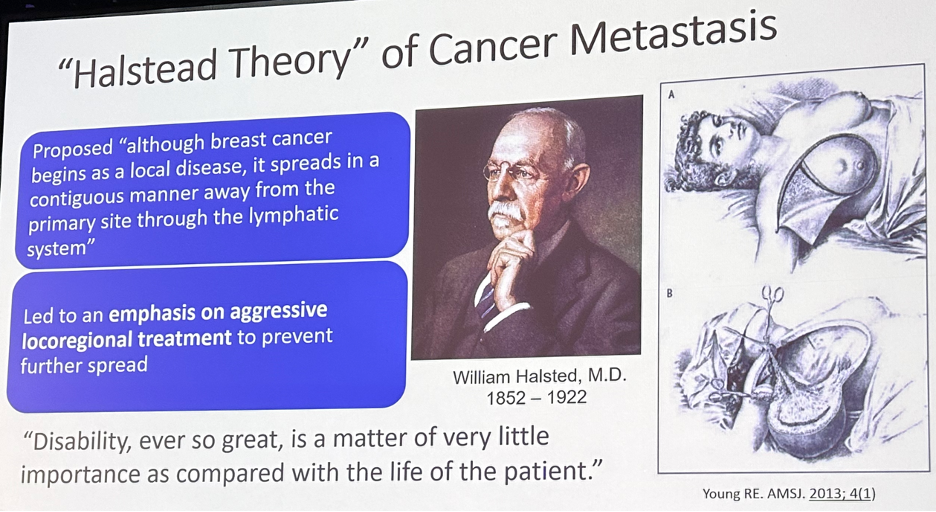
Mirroring our surgical colleagues, urologic oncology at that time trended towards more extensive surgical resections predicated upon observations of anatomic extent of cancer spread, particularly to regional lymph nodes. These more extensive resections were made possible with advances in anesthesia, antibiotics, use of blood products, peri-operative management, and critical care.
Next, we observed Dr. Paget’s “Seed and Soil” theory of metastasis. This theory dictated that “the pattern of metastatic spread is not random, and cancer cells exhibit preferences when metastasizing to organs... the spread of tumor cells is governed by interaction and cooperation between the cancer cells (seed) and the host organ (soil)”. An obvious example of this in urologic oncology is prostate cancer’s predilection for metastasis to the bones.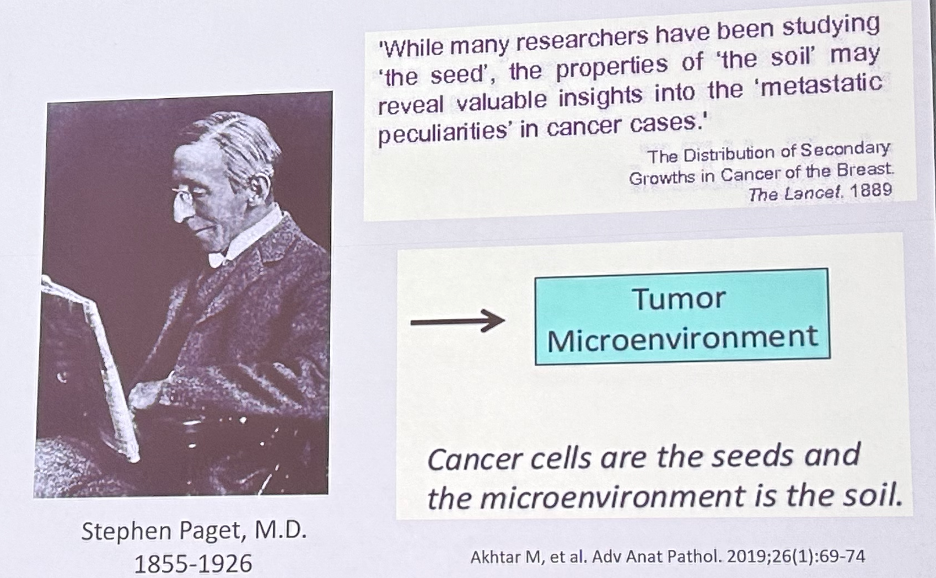
As has been observed in other surgical disciplines, urologic oncologists have spearheaded the reduction in the extent of surgical resection, of which there have been many examples. In 2019, Gschwend et al. published the results of a prospective randomized trial of 401 patients who were randomized to extended versus limited lymph node dissection following radical cystectomy.1 While performance of an extended lymph node dissection increased the median lymph node yield (19 to 31), this was not associated with significant improvements in oncologic outcomes (recurrence-free, cancer-specific, or overall survival), with the additional cost of worse Clavien grade 3 or higher lymphoceles in the extended group (8.6% versus 3.4%). More recently the initial results of the SWOG S1011 trial were presented at ASCO 2023. Similar to the European trial, extended lymph node dissection, as compared to a standard template dissection, did not improve disease-free survival (HR: 1.10, p=0.40) or overall survival (HR: 1.15, p=0.29), with more frequent adverse events, including 90-day mortality rates (6.5% versus 2.4%). These results taken in total have led to the widespread de-escalation from an extended to standard lymph node dissection approaches at the time of radical cystectomy.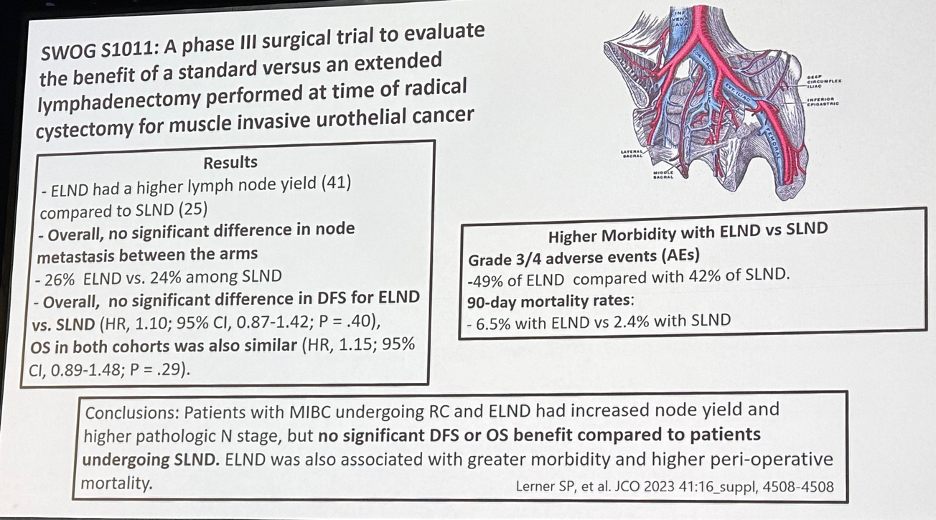
A similar paradigm has been observed in the prostate cancer space. In 2021, Touijer et al. demonstrated that there was no difference in recurrence rates following extended versus limited node dissections during radical prostatectomy.2 As such, there has been a shift away from performing an extended dissection, even in high-risk patients, with future trials focusing on comparing extended to no lymph node dissections.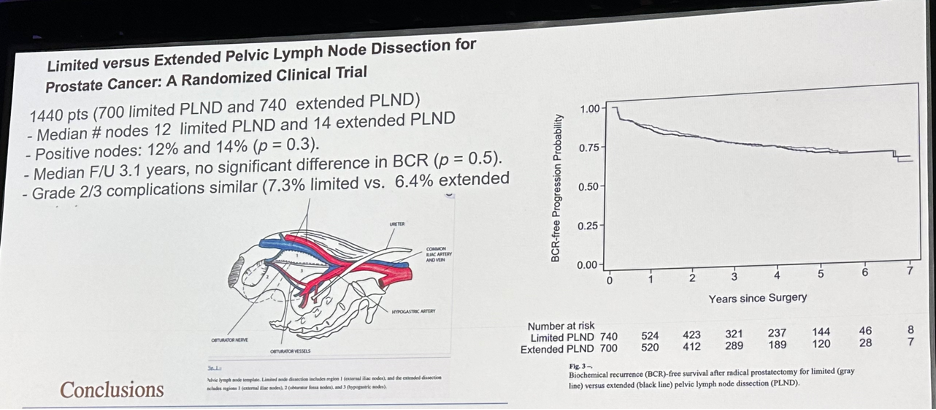
What about extirpative surgery at the time of a female radical cystectomy? In well-selected patients, there has been a recent push to spare female reproductive organs to minimize post-operative morbidity. This has been reflected in the most recent iteration of the AUA/SUO guidelines:
Statement 11: “When performing a standard radical cystectomy with curative intent, clinicians should remove the bladder, prostate, and seminal vesicles in males; clinicians should remove the bladder in females and should consider removal of adjacent reproductive organs based on individual disease characteristics and need to obtain negative margins. (Clinical Principle)”
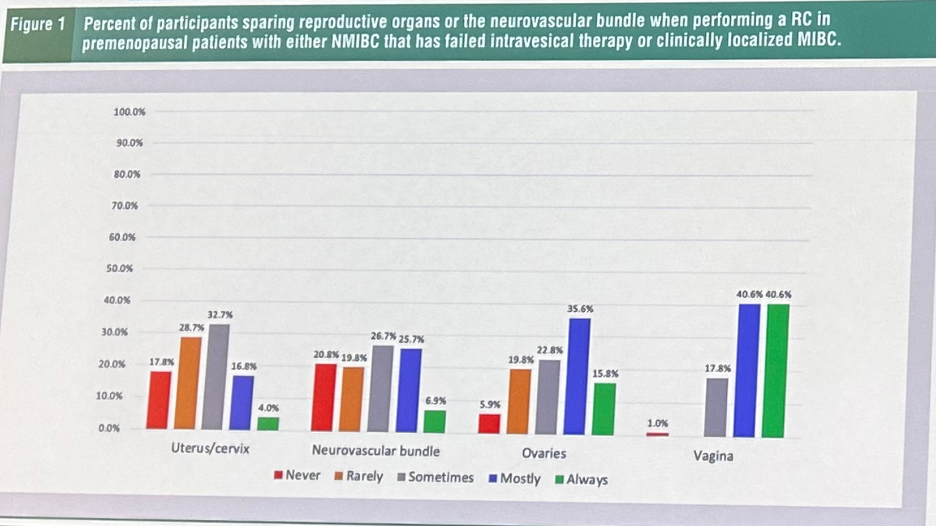
But this unfortunately has not been necessarily reflected in the current state of practice. In a cross-sectional survey of 101 US urologic oncologists aimed at assessing provider practice patterns regarding female reproductive organ-sparing and nerve-sparing radical cystectomy (RC) in pre- and postmenopausal patients with organ-confined disease, the following results were obtained:3
- 2% routinely resect the uterus/cervix
- 3% routinely resect the NVB
- 5% routinely resect the ovaries
- 8% routinely resect a portion of the vagina
What about de-escalation of treatment where appropriate? This has been best reflected in the widespread adoption of active surveillance for the management of low-risk prostate cancer. While we have noticed improvement in the uptake of active surveillance in the US from 27% to 60% in 2021 (AUA Quality [AQUA] registry), approximately 40% of patients with low-risk disease are still receiving definitive therapy at the time of diagnosis.4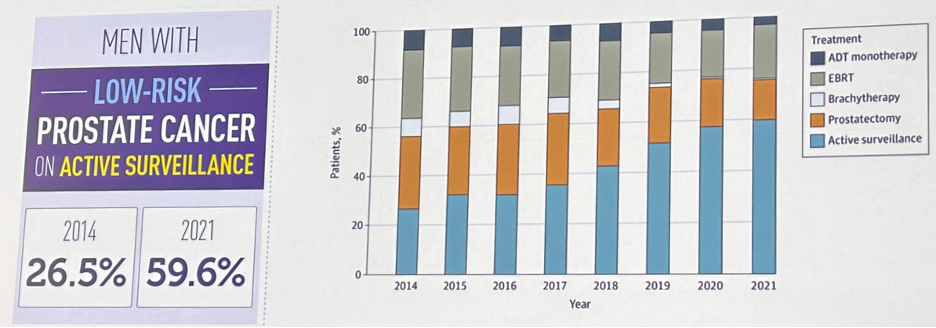
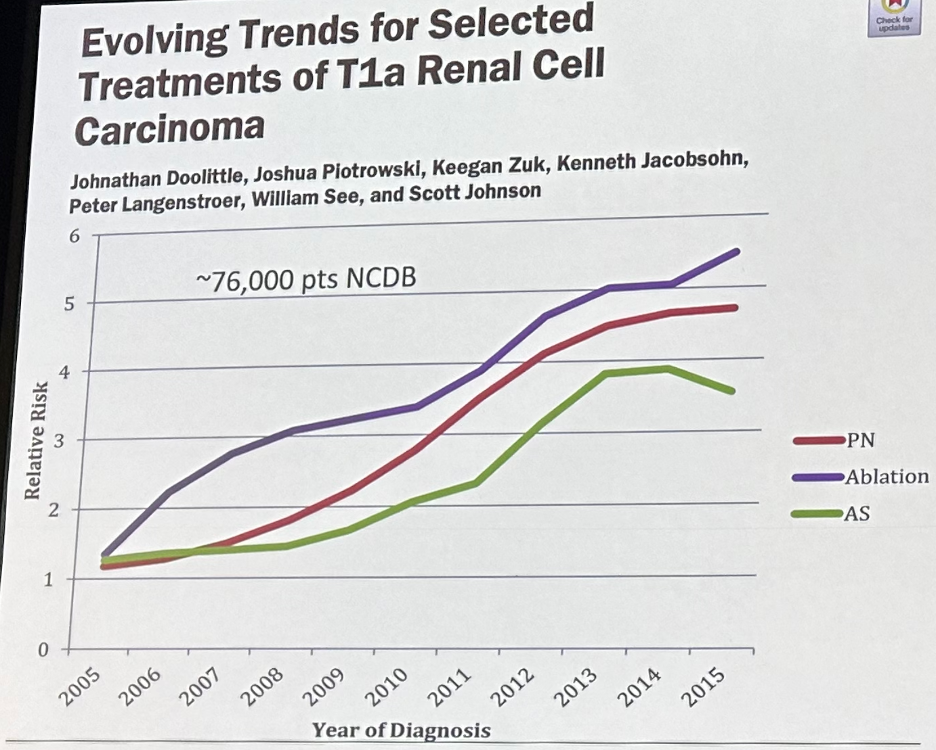
There has similarly been an increased uptake of partial nephrectomy, thermal ablation, and active surveillance for cT1a small renal masses, sparing many patients, particularly those who are elderly with significant comorbidities, the morbidity and sequalae of radical nephrectomy.5
Another important ‘de-escalative’ advance has been the emergence of bladder sparing with trimodality therapy (i.e., TURBT, chemotherapy, radiation) for select patients with muscle invasive bladder cancer. This has been reflected in the most recent 2020 AUA/ASCO/ASTRO/SUO guidelines for the treatment of non-metastatic muscle invasive bladder cancer:
Statement 21: “For patients with newly diagnosed non-metastatic muscle-invasive bladder cancer who desire to retain their bladder, and for those with significant comorbidities for whom radical cystectomy is not a treatment option, clinicians should offer bladder preserving therapy when clinically appropriate. (Clinical Principle)”
Statement 25: “For patients with muscle-invasive bladder cancer who have elected multimodal bladder preserving therapy, clinicians should offer maximal transurethral resection of bladder tumor, chemotherapy combined with external beam radiation therapy, and planned cystoscopic re-evaluation. (Strong Recommendation; Evidence Level: Grade B).”
These recommendations have been supported by numerous recent publications, including that by Zlotta et al. recently published in the The Lancet Oncology that demonstrated that trimodality therapy was non-inferior to radical cystectomy for well-selected patients with muscle invasive bladder cancer.6
Another important advance for the urologic oncologist has been the emergence of minimally invasive surgical approaches. This has been most noticeable for radical prostatectomies, and by 2010, minimally invasive approaches to radical prostatectomy overtook open approaches in both the United States and the United Kingdom.7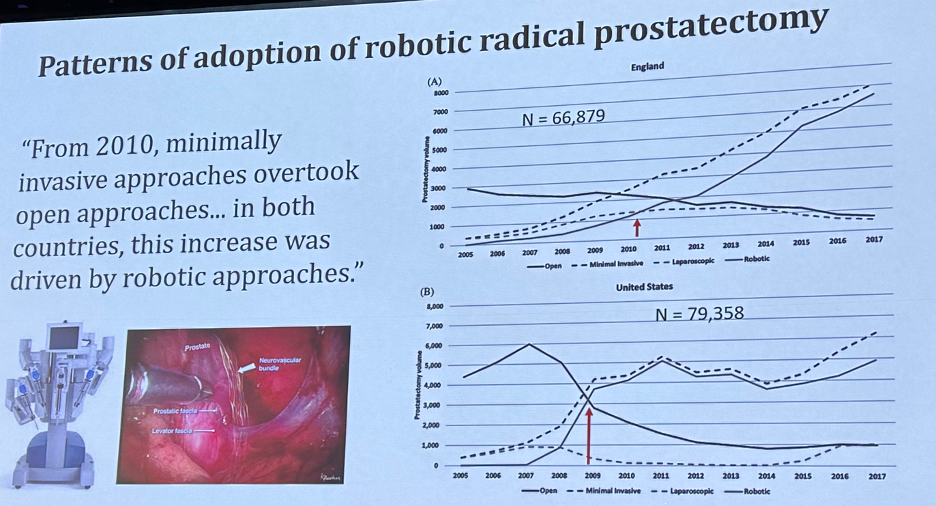
This was soon followed by the adoption of robotic approaches to radical cystectomies, with the phase 3 RAZOR non-inferiority trial demonstrating that robotic cystectomy was non-inferior to open cystectomy for 2-year progression-free survival.8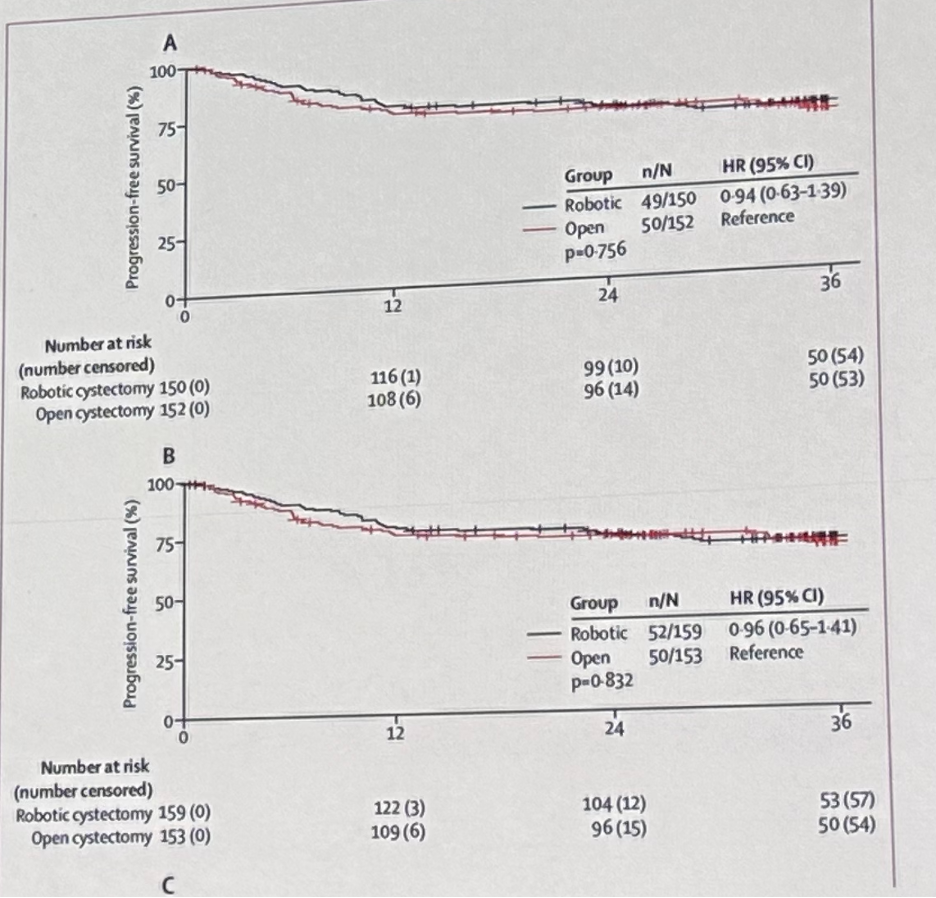
More recently Catto et al. demonstrated that robot-assisted radical cystectomy with intracorporeal urinary diversion, compared to an open radical cystectomy, resulted in a statistically significant increase in days alive and out of hospital over 90 days, with similar oncologic outcomes.9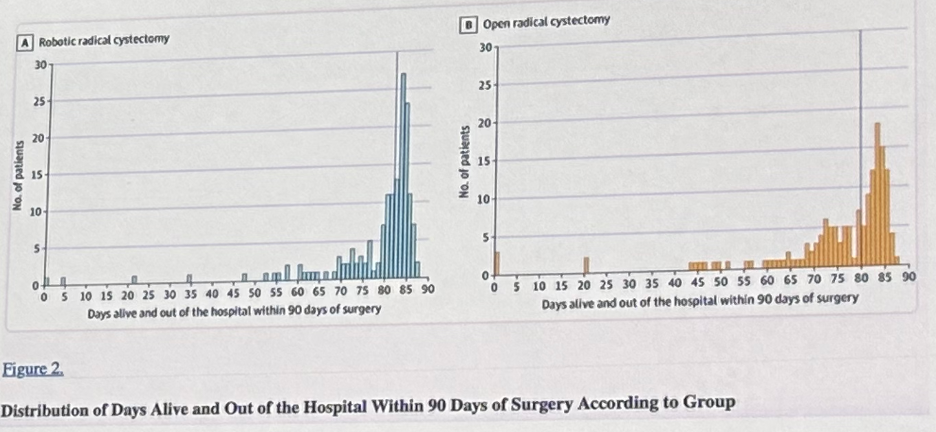
The results of these studies have provided additional support in favor of robotic-assisted approaches to radical cystectomy, and these have been reflected in the increased utilization of this approach in both North America and Europe. In an international, multicenter collaboration that included 2,401 patients from 12 US and European centers, Zamboni et al. demonstrated that between 2006 and 2018, 49.5% of patients underwent robot-assisted and 51.5% an open radical cystectomy. Robotic-assisted radical cystectomy became the most commonly performed procedure in contemporary patients, with an increase from 29% in 2006 – 2008 to 54% in 2015 – 2018 (p< 0.001).10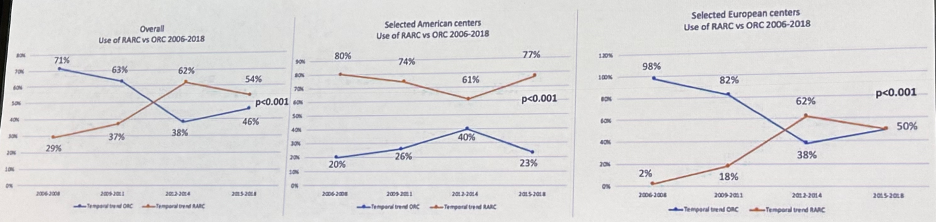
Another important development has been the evolution of the urologic oncologist to become the orchestrator of the multidisciplinary care model: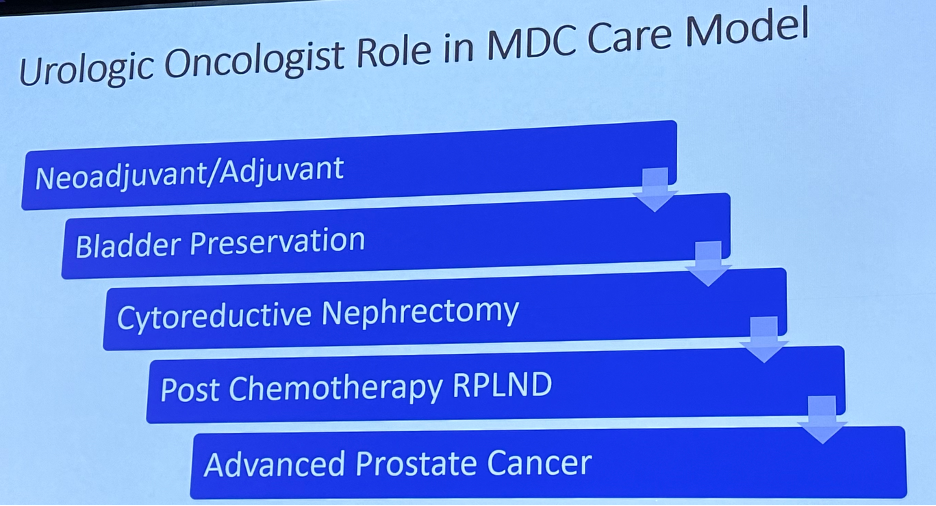
Since the publication of the SWOG trial by Grossman et al. in 2003, neoadjuvant cisplatin-based chemotherapy has emerged as the standard of care option prior to radical cystectomy. This approach has been demonstrated to provide an overall survival benefit of 5 – 7% at 5 years.11,12 Following the diagnosis of muscle invasive disease on a TURBT, it is often incumbent on the urologist to send the referral to their medical oncologist for neoadjuvant therapy consideration. While the utilization of neoadjuvant chemotherapy has increased from 23% to 32% between 2011 and 2015,13 it remains clear that a significant proportion of eligible patients do not receive neoadjuvant chemotherapy.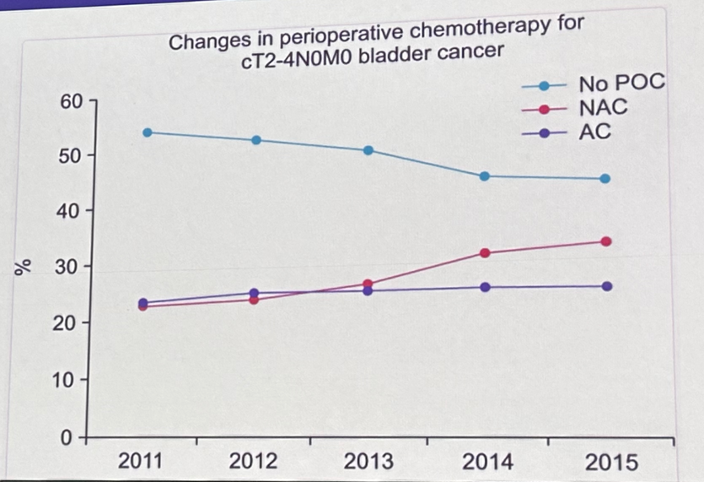
More recently, we have witnessed the emergence of nivolumab in the adjuvant setting for muscle-invasive urothelial carcinoma, following the publication of CheckMate-274.14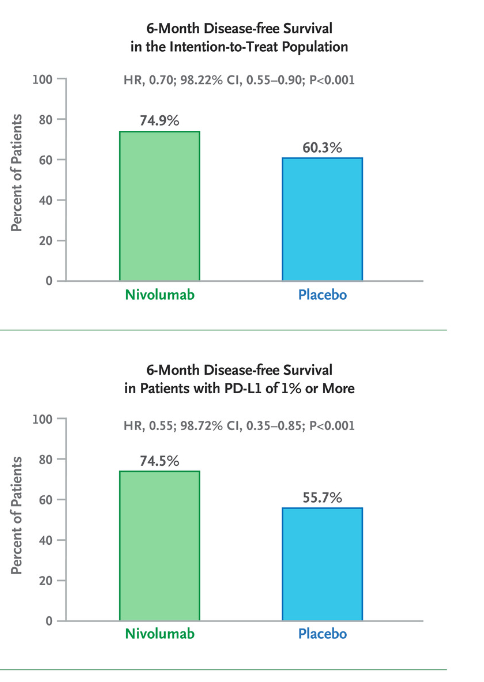
Analogously in the locoregionally advanced clear cell RCC setting, adjuvant pembrolizumab has emerged as a treatment option with the publication of the KEYNOTE-564 trial,15 with a recent press release confirming that adjuvant pembrolizumab is associated with an overall survival benefit in this setting.
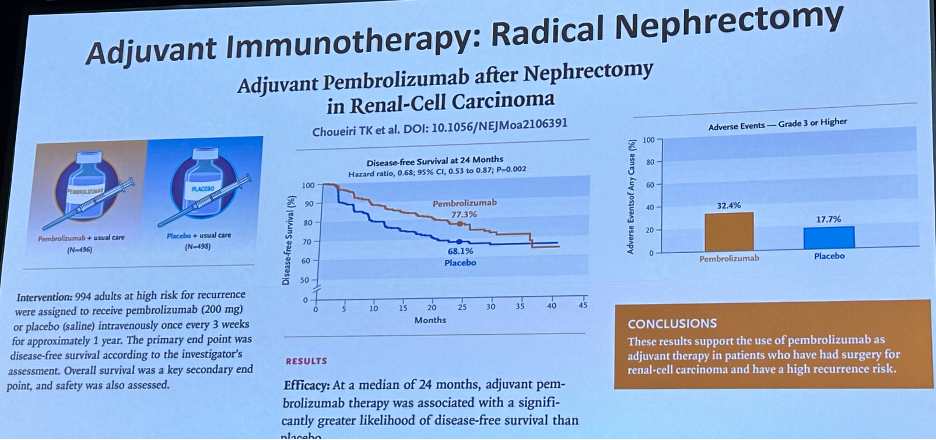
Platinum-based chemotherapy combinations have also emerged as an adjuvant treatment option for patients with upper tract urothelial carcinoma following the publication of the phase 3 POUT trial.16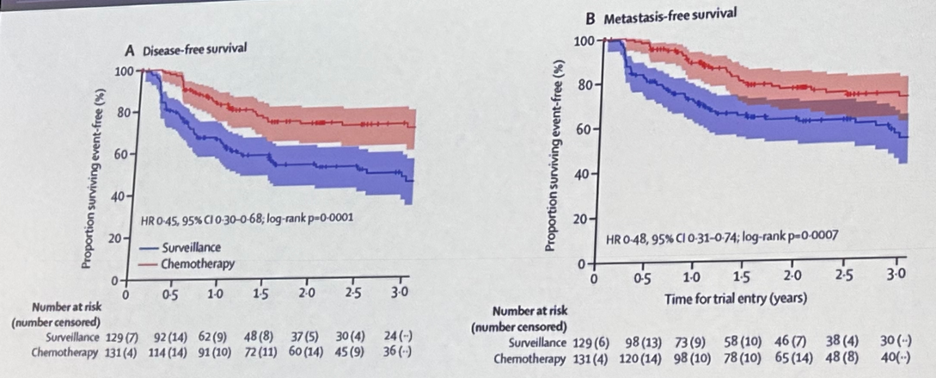
Dr. Cookson highlighted that given the preponderance of this multi-disciplinary evidence, significant efforts have been made by the AUA guidelines committee, many of which have been chaired by Dr. Cookson himself, which has culminated in 38 guidelines statements:
- Early evaluation and counseling
- Biochemical recurrence
- Metastatic castrate-sensitive prostate cancer
- Non-metastatic castrate-resistant prostate cancer
- Metastatic CRPC
- Bone health
As noted above, many of these have been in the prostate cancer disease space, where we have witnessed an ‘explosion’ of FDA-approved agents for patients with advanced prostate cancer.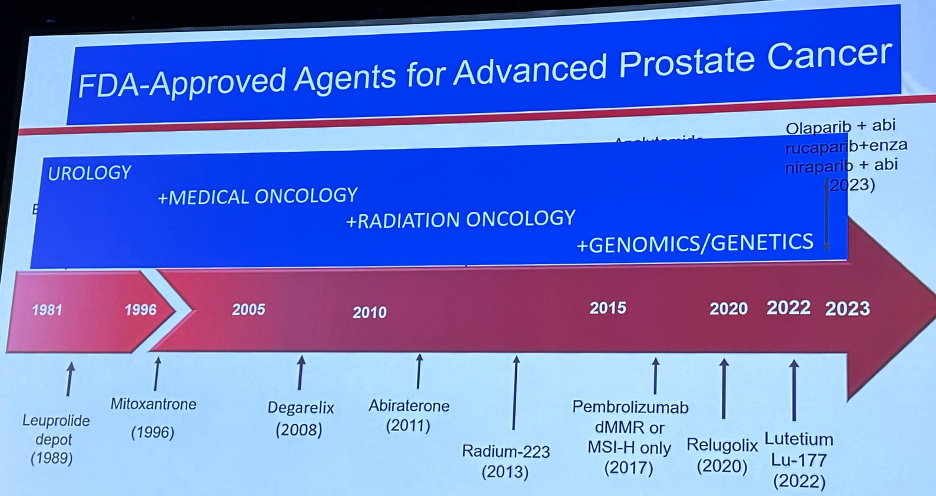
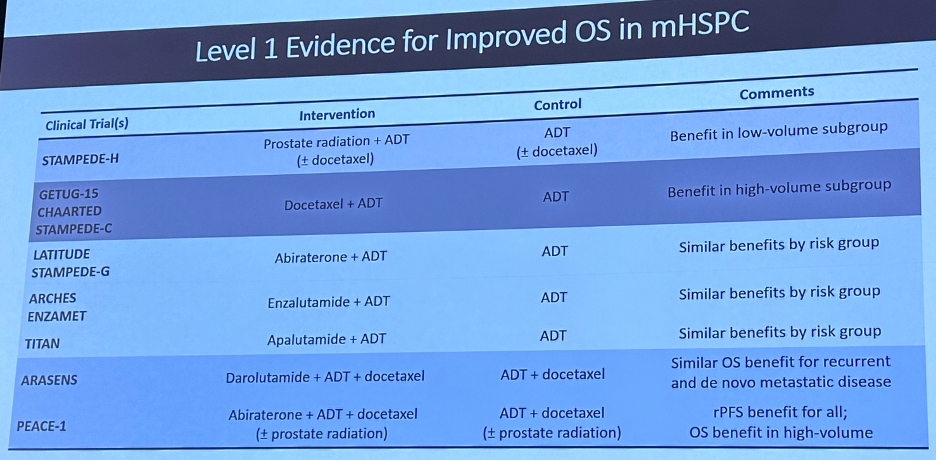
Despite these important advances, with doublet and triplet therapy options firmly established as standard of care options in the metastatic hormone-sensitive setting, we see that real-world treatment patterns do not adequately reflect these recommendations with a considerable implementation gap present.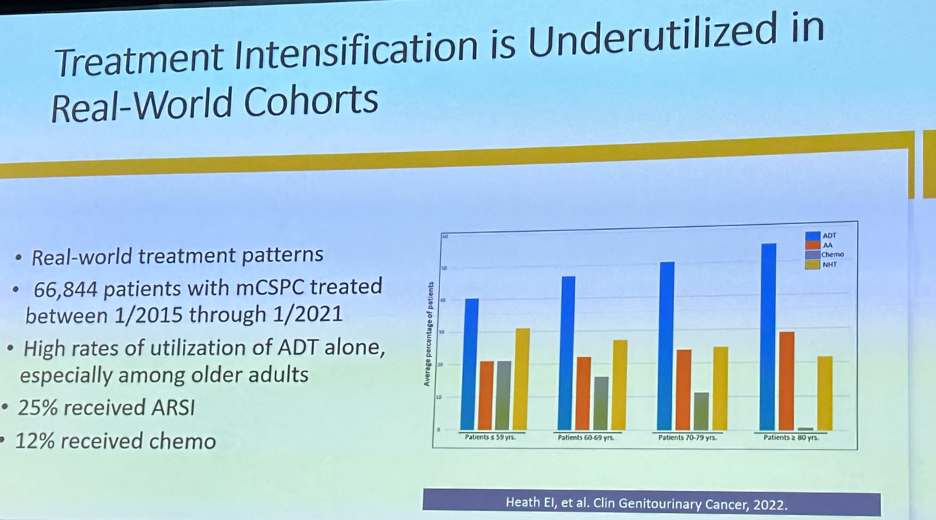
But as a surgical discipline, Dr. Cookson emphasized that we need to still do better in many disease spaces. As systemic therapy options have been moved up the disease spectrum, we have noted a greater stepwise survival benefit as summarized below: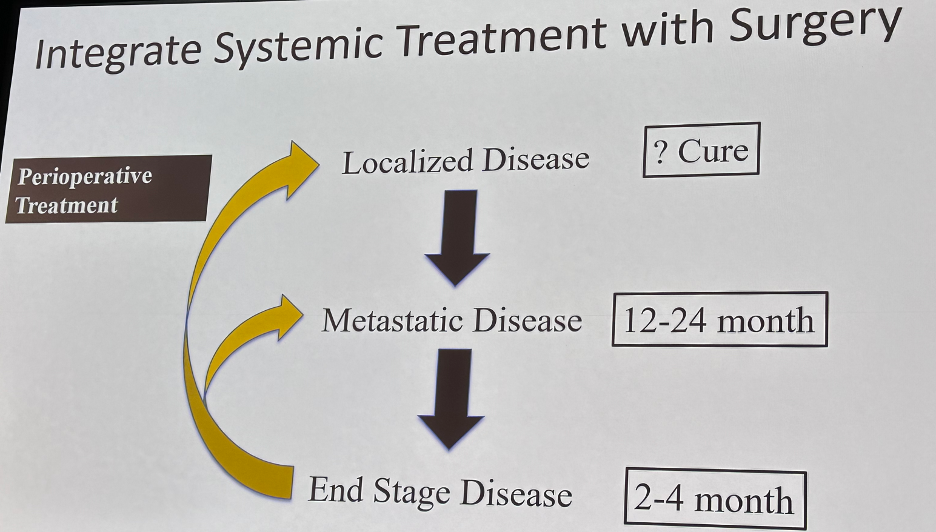
Thus, one of clear the next steps for our surgical field is to improve upon neoadjuvant therapy options prior to radical prostatectomy for higher risk prostate cancer patients. Many of the trials in this space have shown improved pathologic outcomes, but, to date, these have not translated into improved survival outcomes.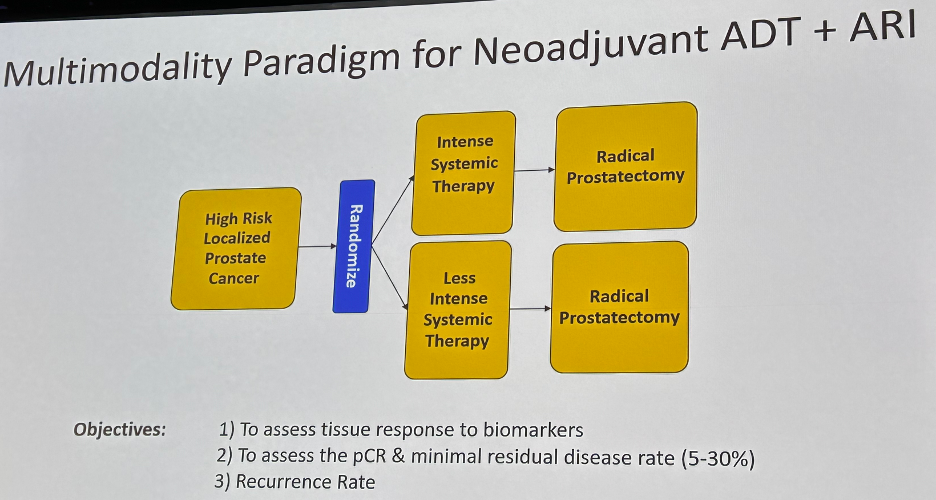
Finally, we have seen the emergence of precision medicine in our field. This has been most notable in the advanced prostate cancer space, particularly following the seminal publication by Pritchard et al. that described the germline mutational landscape in both localized and advanced prostate cancer.17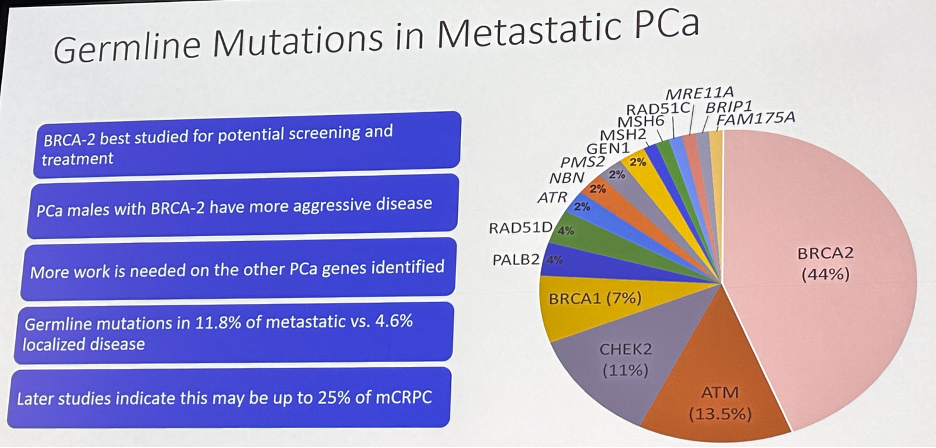
Dr. Cookson concluded by thanking his mentors along the way and offered his perspective on the individual surgeon’s evolution through their career, highlighting one’s evolving priorities and responsibilities along the way.
Presented by: Michael S. Cookson, MD, MMHC, Professor and Chair, Department of Urology, Oklahoma University College of Medicine, Oklahoma City, OK
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Society of Urologic Oncology (SUO) Annual Meeting, Washington, D.C., Tues, Nov 28 – Fri, Dec 1, 2023.
References:
- Gschwend JE, Heck MM, Lehmann J, et al. Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. Eur Urol. 2019;75(4):604-611.
- Touijer KA, Sjoberg DD, Benfante N, et al. Limited versus Extended Pelvic Lymph Node Dissection for Prostate Cancer: A Randomized Clinical Trial. Eur Urol Oncol. 2021;4(4):532-539.
- Gupta N, Kucirka L, Semerjian A, et al. Practice Patterns Regarding Female Reproductive Organ-Sparing and Nerve-Sparing Radical Cystectomy Among Urologic Oncologists in the United States. Clin Genitourin Cancer. 2023;21(4):E236-241.
- Cooperberg MR, Meeks W, Fang R, et al. Time Trends and Variation in the Use of Active Surveillance for Management of Low-risk Prostate Cancer in the US. JAMA Netw Open. 2023; 6(3):e231439.
- Doolittle J, Piotrowski J, Zuk K, et al. Evolving Trends for Selected Treatments of T1a Renal Cell Carcinoma. Urology. 2019;132:136-142.
- Zlotta AR, Ballas LK, Niemierko A, et al. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 2023;24(6):669-681.
- Maynou L, Mehtsun WT, Serra-Sastre V, Papanicolas I. Patterns of adoption of robotic radical prostatectomy in the United States and England. Health Serv Res. 2021;56 Suppl 3(Suppl 3):1441-1461.
- Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet. 2018;391(10139):2525-2536.
- Catto JWF, Khetrapal P, Ricciardi F, et al. Effect of Robot-Assisted Radical Cystectomy With Intracorporeal Urinary Diversion vs Open Radical Cystectomy on 90-Day Morbidity and Mortality Among Patients With Bladder Cancer: A Randomized Clinical Trial. JAMA. 2022;327(21):2092-2103.
- Zamboni S, Soria F, Mathieu R, et al. Differences in trends in the use of robot-assisted and open radical cystectomy and changes over time in peri-operative outcomes among selected centres in North America and Europe: an international multicentre collaboration. BJU Int. 2019;124(4):656-664.
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349(9):859-66.
- Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 2003;361(9373):1927-34.
- McFerrin C, Davaro F, May A, et al. Trends in utilization of neoadjuvant and adjuvant chemotherapy for muscle invasive bladder cancer. Investig Clin Urol. 2020;61(6):565-572.
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2021;384:2102-2114.
- Choueiri TK, Tomczak P, Park SH, et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med. 2021;485(8):683-694.
- Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomized controlled trial. Lancet. 2020;395(10232):1268-1277.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453


