(UroToday.com) The 2023 Society of Urologic Oncology (SUO) annual meeting held in Washington, D.C. between November 28th and December 1st, 2023, hosted a prostate cancer session. Dr. Heather Cheng presented the SWOG 2210 trial of neoadjuvant carboplatin for patients with high-risk, localized prostate cancer and germline BRCA1/2 mutations.
Patients with localized high risk prostate cancer have an approximately 50% chance of disease progression following radical prostatectomy.1 Germline BRCA1/ 2 mutation carriers with localized prostate cancer treated with curative intent have worse outcomes (e.g., overall and metastasis-free survivals) compared to those who do not.2,3 Neoadjuvant therapy remains a non-standard of care option for higher-risk, clinically localized prostate cancer, but numerous androgen receptor pathway inhibitors are being prospectively evaluated in phase clinical trials (e.g., PROTEUS). BRCA1/2 mutations confer sensitivity to DNA damaging agents, such as platinum chemotherapy and PARP inhibitors. Carboplatin is well-tolerated, available, and almost 50-fold cheaper compared to PARP inhibitors. Neoadjuvant therapy is standard of care treatment for other germline BRCA1/2m-associated cancers (e.g., breast, ovarian cancers). Can such a paradigm be translated to patients with similarly mutated prostate cancers?
The hypothesis for the SWOG 2210 trial is: 12 weeks (4 doses) of neoadjuvant carboplatin followed by radical prostatectomy for patients with localized high or very high-risk prostate cancer and germline BRCA1/2 mutations will result in meaningful pathologic complete response rates.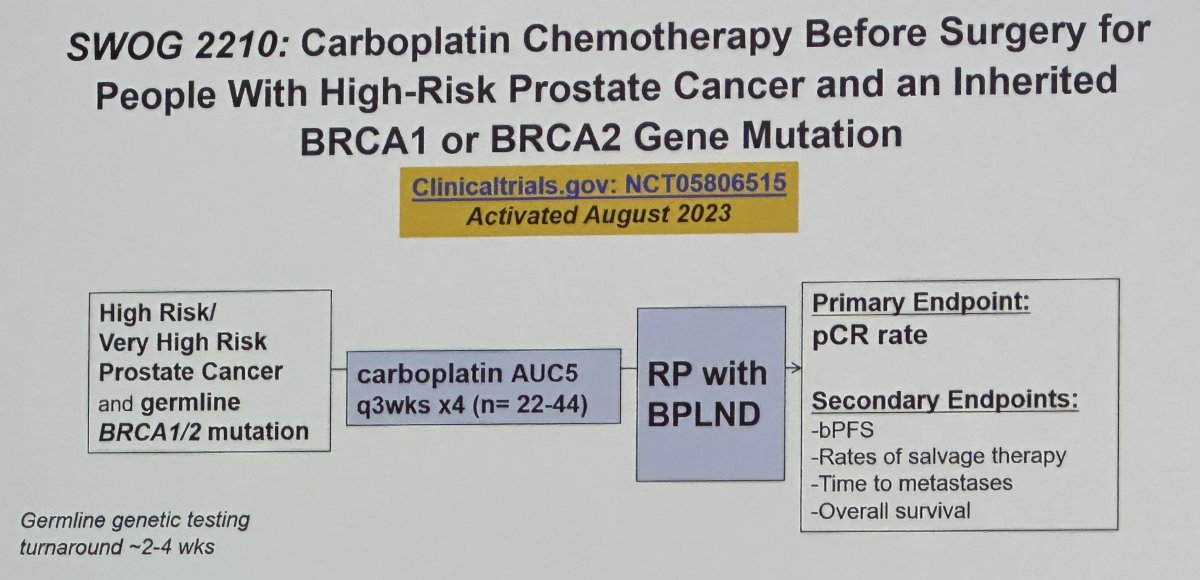
The primary endpoint is pathologic complete response rate, with secondary endpoints including
- Biochemical progression-free survival
- Rates of salvage therapy
- Time to metastases
- Overall survival
The key eligibility criteria are as follow:
- High or very high-risk prostate adenocarcinoma: T3a OR Grade Group 4 or Grade Group 5 or PSA>20 ng/mL (may have initiated up to 90 days ADT prior to randomization)
- No evidence of distant metastatic disease by conventional imaging (PSMA-PET detected metastases permitted if no metastases by conventional imaging and the surgeon deems all PSMA-avid sites resectable)
- Candidate for radical prostatectomy and carboplatin (ECOG 0-1, adequate organ function)
- Documented evidence of germline mutation in BRCA2 or BRCA1 through testing in a chemiluminescent immunoassay (CLIA)-certified lab (local lab report sufficient for eligibility, prior knowledge of mutation is allowed and encouraged)
Dr. Cheng emphasized that urologic oncologists will be essential for the exciting future of precision management and will be key partners for trials of neoadjuvant therapies.
Presented by: Heather H. Cheng, MD, PhD, Associate Professor, Division of Hematology and Oncology, Department of Medicine, University of Washington, Seattle, WA
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Society of Urologic Oncology (SUO) annual meeting held in Washington, D.C. between November 28th and December 1st, 2023
References:
- Kane CJ, Presti Jr JC, Amling CL, et al. Changing nature of high risk patients undergoing radical prostatectomy. J Urol. 2007;177(1):113-117.
- Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748-1757.
- Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. Eur Urol. 2015;68(2):186-193.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453.


